

Chinese Journal of Organic Chemistry >
Design, Synthesis and Anti-proliferative Activity of Novel 5,6-Dihydro-6-alkyl-2-pyrone Analogues
Received date: 2016-12-02
Revised date: 2016-12-30
Online published: 2017-01-17
Supported by
Project supported by the Program for Science & Technology Innovation Talents of Henan Province (No. 14HASTIT0031).
In order to investigate whether the modification at the double bonds in the pyrone skeleton would affect their biological activity. Based on the 5,6-dihydro-6-alkyl-2-pyrone goniothalamin, a series of new compounds 5, 6 and 8 were designed and synthesized, which were obtained by modification at the unsubstituted double bonds. Their cell proliferation inhibition activities against human liver cancer (7721), human lung cancer (A549), human esophageal cancer (EC-109), human gastric cancer (MGC-803) cell lines were assessed by thiazolyl blue tetrazolium bromide (MTT) method. Most of synthetic compounds lost their bioactivities via a comparision with positive control but 6-(benzyloxy)methyl-3-methyl-4-(2,3,4,5-tetra- fluorobenzamine)-5,6-dihydro-2H-pyran-2-one (6d). The results indicate that the unsaturated double bond is a key functional group for the bioactivity of these compounds and it is possible to keep their anti-cancer proliferation activity and reduce their toxicity by modification of the unsaturated double bond in 5,6-dihydro-6-alkyl-2-pyrones.
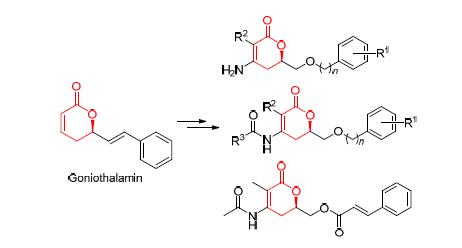
Key words: α,β-unsaturated δ-lactone; synthesis; cancer; antiproliferative activity
Xu Haiwei , Jia Shilong , Xie Xiaoping , Luo Jiao , Wang Shu . Design, Synthesis and Anti-proliferative Activity of Novel 5,6-Dihydro-6-alkyl-2-pyrone Analogues[J]. Chinese Journal of Organic Chemistry, 2017 , 37(4) : 902 -907 . DOI: 10.6023/cjoc201612007
[1] Jong, R.; Mulder, N.; Uges, D. Brit. J. Cancer. 1999, 79, 882.
[2] Barros, M. E.; Freitas, J. C.; Oliveira, J. M. Eur. J. Med. Chem. 2014, 76, 291.
[3] Seyed, M. A.; Jantan, I.; Bukhari, S. N. A. BioMed Res. Int. 2014, 2014, 536508.
[4] Sun, Q. X.; Carrasco, Y. P.; Hu, Y. C. P. Natl. Acad. Sci. U. S. A. 2013, 110, 1303.
[5] Sandra, T. G.; Concepcion, V.; Santiago, D. O. Arch. Pharm. Chem. Life Sci. 2015, 348, 541.
[6] Raju, A.; Reddy, A. Y.; Sabitha, G. Tetrahedron Lett. 2015, 56, 5474.
[7] Luiz, F. T. N.; Carolina, M. A.; Karin, J. P. R. Chem. Med. Chem. 2015, 10, 1687.
[8] Vilanova, C.; Diaz-Oltra, S.; Murga, J. J. Med. Chem. 2014, 57, 10391.
[9] Marucci, C.; Christodoulou, M. S.; Pieraccini, S. Eur. J. Org. Chem. 2016, 11, 2029.
[10] Benedekovic, G.; Kovacevic, I.; Popsavin, M. Bioorg. Med. Chem. Lett. 2016, 26, 3318.
[11] Li, X. C.; Guo, Z. Y.; Deng, Z. S. Rec. Nat. Prod. 2015, 9, 503.
[12] He, H. Y.; Ratnayake, A. S.; Janso, J. E. J. Nat. Prod. 2014, 77, 1864.
[13] Marco, J. A.; García-Pla, J.; Carda, M. Eur. J. Med. Chem. 2011, 46, 1630.
[14] Kim, J. H.; Lee, S. J.; Org. Lett. 2011, 13, 1350.
[15] Kim, J. H.; Shin, H.; Lee, S. G. J. Org. Chem. 2012, 77, 1560.
[16] Cao, X. F.; Sun, T. T.; Ke, S. Y. Chin. J. Org. Chem. 2010, 30, 1113 (in Chinese).
(曹秀芳, 孙婷婷, 柯少勇, 有机化学, 2010, 30, 1113.)
[17] Koziol, A.; Lendzion-Paluch, A.; Manikowski, A. Org. Proc. Res. Dev. 2013, 17, 869.
[18] Zhao, J. Y.; Zheng, Z. H.; Huang, Q. F.; Deng, J. G.; Zhu, J.; Wang, Q. W. Chin. J. Org. Chem. 2016, 36, 648 (in Chinese).
(赵剑阳, 郑紫华, 黄晴菲, 邓金根, 朱槿, 王启卫, 有机化学, 2016, 36, 648.)
[19] Pospisil, J.; Marko, I. E. J. Am. Chem. Soc. 2007, 129, 3516.
[20] Ramesh, D.; Rajaram, S.; Peddikotla, P. Helv. Chim. Acta 2011, 94, 1226.
[21] AnkiReddy, S.; AnkiReddy, P.; Sabitha, G. Org. Biomol. Chem. 2015, 13, 10487.
/
| 〈 |
|
〉 |