

Chinese Journal of Organic Chemistry >
Synthesis and Cytotoxic Activity of Novel Hybrid Compounds between Indolo[b]tetrahydrofuran and Imidazolium Salts
Received date: 2016-10-28
Revised date: 2017-01-12
Online published: 2017-01-20
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21662043, 21462049, U1402227, 21332007), the Program for Changjiang Scholars and Innovative Research Team in University (No. IRT13095), the Natural Science Foundation of Yunnan Province (No. 2013FA028).
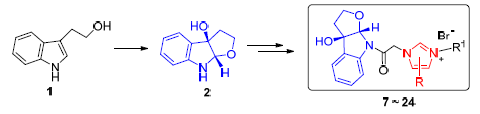
A series of novel hybrid compounds between indolo[b]tetrahydrofuran and imidazolium salts were prepared from tryptophol by four steps of Sharpless epoxidation, amidation, coupling and salt formation. Their structures were confirmed by 1H NMR, 13C NMR, HRMS and X-ray crystallographic analysis. These compounds were evaluated in vitro against a panel of human tumor cell lines. The results showed that 1-((3aR,8aS)-3,3a-dihydro-3a-hydroxy-2H-furo[2,3-b]indol-8(8aH)-yl)etha-none-3-(2-(naphthalen-2-yl)-2-oxoethyl)-5,6-dimethyl-1H-benzo[d]-imidazol-3-ium bromide (20) and 1-((3aR,8aS)-3,3a-di-hydro-3a-hydroxy-2H-furo[2,3-b]indol-8(8aH)-yl)ethanone-3-(2-naphthylmethyl)-5,6-dimethyl-1H-benzo[d]imidazol-3-ium bromide (22) exhibited higher inhibitory activity selectively against SMMC-7721, MCF-7 and SW480 cell lines compared with DDP. In particular, 1-((3aR,8aS)-3,3a-dihydro-3a-hydroxy-2H-furo[2,3-b]indol-8(8aH)-yl)ethanone-3-(2-bromobenzyl)-5,6-dimethyl-1H-benzo[d]imidazol-3-ium bromide (24) was more selective to SW-480 cell lines with IC50 values 2.0-fold lower than DDP.
Liu Zhengfen , Zhang Chaobo , Duan Shengzu , Liu Yang , Chen Wen , Li Yan , Zhang Hongbin , Yang Xiaodong . Synthesis and Cytotoxic Activity of Novel Hybrid Compounds between Indolo[b]tetrahydrofuran and Imidazolium Salts[J]. Chinese Journal of Organic Chemistry, 2017 , 37(6) : 1506 -1515 . DOI: 10.6023/cjoc201610043
[1] Pan, C. X.; Guan, Y. F.; Zhang, H. B. Chin. J. Org. Chem. 2012, 32, 1116 (in Chinese).(潘成学, 关一富, 张洪彬, 有机化学, 2012, 32, 1116.)
[2] Wang, X. Q.; Li, Y.; Yang, X. D.; Zhang, H. B. Chin. J. Org. Chem. 2015, 35, 1276 (in Chinese).(汪学全, 李艳, 羊晓东, 张洪彬, 有机化学, 2015, 35, 1276.)
[3] (a) Suzuki, T.; Choi, J. H.; Kawaguchi, T.; Yamashita, K.; Morita, A.; Hirai, H.; Nagai, K.; Hirose, T.; Omura, S.; Sunazuka, T.; Kawagishi, H. Bioorg. Med. Chem. Lett. 2012, 22, 4246.
(b) Chaudhaery, S. S.; Roy, K. K.; Shakya, N.; Saxena, G.; Sammi, S. R.; Nazir, A.; Nath, C.; Saxena, A. K. J. Med. Chem. 2010, 53, 6490.
(c) Luo, W.; Yu, Q. S.; Kulkarni, S. S.; Parrish, D. A.; Holloway, H. W.; Tweedie, D.; Shafferman, A.; Lahiri, D. K.; Brossi, A.; Greig, N. H. J. Med. Chem. 2006, 49, 2174.
(d) Gavuzzo, E.; Pomponi, M. J. Biochem. Mol. Toxicol. 2002, 16, 64.
[4] (a) Hayashi, M.; Kim, Y.-P.; Takamatsu, S.; Enomoto, A.: Shinose, M.; Takahashi, Y.; Tanaka, H.; Komiyama, K.; Ohmura, S. J. Antibiot. 1996, 49, 1091.
(b) Takamatsu, S.; Kim, Y.-P.; Enomoto, A.; Hayashi, M.; Tanaka, H.; Komiyama, K.; Ohmura, S. J. Antibiot. 1997, 50, 1069.
[5] Hayashi, M.; Rho, M.-C.; Enomoto, A.; Fukami, A.; Kim, Y.-P.; Kikuchi, Y.; Sunazuka, T.; Hirose, T.; Komiyama, K.; Ohmura, S. Proc. Natl. Acad. Sci. 2002, 99, 14728.
[6] (a) Strassmann, G.; Masui, Y.; Chizzonite, R.; Fong, M. J. Immunol. 1993, 150, 2341.
(b) Zhang, X. G.; Bataille, R.; Jourdan, M.; Saeland, S.; Banchereau, J.; Mannoni, P.; Klein, B. Blood 1990, 76, 2599.
[7] Wan, L.; Tius, M. A. Org. Lett. 2007, 9, 647.
[8] (a) Vlahakis, J. Z.; Lazar, C.; Crandall, I. E.; Szarek, W. A. Bioorg. Med. Chem. 2010, 18, 6184.
(b) Dominianni, S. J.; Yen, T. T. J. Med. Chem. 1989, 32, 2301.
(c) Pardin, C.; Schmitzer, A. R.; Leclercq, L. Chem. Eur. J. 2010, 16, 4686.
[9] (a) Fortuna, C. G.; Barresi, V.; Berellini, G..; Musumarra, G. Bioorg. Med. Chem. 2008, 16, 4150.
(b) Saberi, M. R.; Vinh, T. K.; Yee, S. W.; Griffiths, B. J. N.; Evan, P. J.; Simsons, C. J. Med. Chem. 2006, 49, 1016.
[10] Cui, B.; Zheng, B. L.; He, K.; Zheng, Q. Y. J. Nat. Prod. 2003, 66, 1101.
[11] (a) Zeng, X. H.; Yang, X. D.; Zhang, Y. L.; Qing, C.; Zhang, H. B. Bioorg. Med. Chem. Lett. 2010, 20, 1844.
(b) Yang, X. D.; Zeng, X. H.; Zhang, Y. L.; Qing, C.; Song, W. J.; Li, L.; Zhang, H. B. Bioorg. Med. Chem. Lett. 2009, 19, 1892.
[12] D'hooghe, M.; Mollet, K.; De Vreese, R.; Jonckers, T. H. M.; Dams, G.; De Kimpe, N. J. Med. Chem. 2012, 55(11), 5637.
[13] Viegas-Junior, C.; Danuello, A.; Bolzani, V. S.; Barreiro, E. J.; Fraga, C. A. M. Curr. Med. Chem. 2007, 14(17), 1829.
[14] (a) Walsh, J. J.; Bell, A. Curr. Pharm. Des. 2009, 15, 2970.
(b) Zhu, P. F.; Zhao, J. F.; Yang, X. D.; Zhang, H. B. Chin. J. Org. Chem. 2014, 34, 1167 (in Chinese).(朱培芳, 赵静峰, 羊晓东, 张洪彬, 有机化学, 2014, 34, 1167.)
[15] (a) Zhou, B.; Liu, Z. F.; Deng, G. G.; Chen, W.; Li, M. Y.; Yang, L. J.; Li, Y.; Yang, X. D., Zhang, H. B. Org. Biomol. Chem. 2016, 14, 9423.
(b) Zhou, Y. J.; Duan, K. Y.; Zhu, L.; Liu, Z. F.; Zhang, C. G.; Yang, L. J.; Li, M. Y.; Zhang, H. B., Yang, X. D. Bioorg. Med. Chem. Lett. 2016, 26, 460.
(c) Liu, L. X.; Wang, X. Q.; Zhou, B.; Yang, L. J.; Li, Y.; Zhang, H. B.; Yang, X. D. Sci. Rep. 2015, 5, 13101.
(d) Xu, X. L.; Yu, C. L.; Chen, W.; Li, Y. C.; Yang, L. J.; Li, Y.; Zhang, H. B.; Yang, X. D. Org. Biomol. Chem. 2015, 13, 1550.
(e) Xu, X. L.; Wang, J.; Yu, C. L.; Chen, W.; Li, Y. C.; Li, Y.; Zhang, H. B.; Yang, X. D. Bioorg. Med. Chem. Lett. 2014, 24, 4926.
(f) Sun, C. J.; Chen, W.; Li, Y.; Liu, L. X.; Wang, X. Q.; Li, L. J.; Zhang, H. B.; Yang, X. D. RSC Adv., 2014, 4, 16312.
(g) Liu, L. X.; Wang, X. Q.; Yan, J. M.; Li, Y.; Sun, C. J.; Chen, W.; Zhou, B.; Zhang, H. B.; Yang, X. D. Eur. J. Med. Chem. 2013, 66, 423.
(h) Wang, X. Q.; Liu, L. X.; Li, Y.; Sun, C. J.; Chen, W.; Li, L.; Zhang, H. B.; Yang, X. D. Eur. J. Med. Chem. 2013, 62, 111.
[16] Tomoyasu, H.; Toshiaki, S.; Daisuke, Y.; Naoto, K.; Tatsuya, S.; Yoshihiro, H.; Isao, K.; Satoshi, O. Tetrahedron 2005, 61, 6015.
[17] CCDC 1509927 and 1509928 contain the supplementary crystallographic data for compounds 11 and 18. These data can be obtained free of charge from The Cambridge Crystallographic Data Center via www.ccdc.cam.ac.uk/data_request/cif.
/
| 〈 |
|
〉 |