

Chinese Journal of Organic Chemistry >
A Practical Synthesis of Trifluoromethanesulfonate Esters
Received date: 2017-01-18
Revised date: 2017-01-20
Online published: 2017-01-20
Supported by
Project supported by the National Natural Science Foundation of China (No. 20902098).
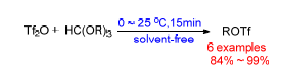
A practical synthesis of trifluoromethanesulfonate esters by reaction of orthoformate esters with triflic anhydride is described. The solvent-free method featured mild condition, short time, high yield, simple operation and broad substrate scope. The in situ generated trifluoromethanesulfonate ester is highly reactive alkylating agent, providing triflate ionic liquid in excellent yield via a one-pot procedure.
Zheng Dongqing , Ma Haiyan , Ding Kai . A Practical Synthesis of Trifluoromethanesulfonate Esters[J]. Chinese Journal of Organic Chemistry, 2017 , 37(6) : 1582 -1584 . DOI: 10.6023/cjoc201701035
[1] (a) Ulibarri, G.; Choret, N.; Bigg, D. C. H. Synthesis 1996, 1286.
(b) Wang, S. F.; Zhang, A. J. Org. Prep. Proced. Int. 2008, 40 (3), 293.
(c) Chen, Z. J.; Xi, H. W.; Lim, K. H.; Lee, J. M. Angew. Chem., Int. Ed. 2013, 52(50), 13392.
(d) Dang, H.; Mailig, M.; Lalic, G. Angew. Chem., Int. Ed. 2014, 53(25), 6473.
(e) Zhao, P.; Yan, X. Y.; Yin, H.; Xi, C. J. Org. Lett. 2014, 16(4), 1120.
[2] (a) Gramstad, T.; Haszeldine, R. N. J. Chem. Soc. 1956, 173. 1.
(b) Chapman, R. D.; Andreshak, J. L.; Herrlinger, S. P.; Shackelford, S. A.; Hildreth, R. A.; Smith, J. P. J. Org. Chem. 1986, 51(20), 3792.
[3] Baraznenok, I. L.; Nenajdenko, V. G.; Balenkova, E. S. Tetrahedron 2000, 56(20), 3077.
[4] Aubert, C.; Begue, J. P. Synthesis 1985, (8), 759.
[5] Beard, C. D.; Baum, K.; Grakausk, V. J. Org. Chem. 1973, 38(21), 3673.
[6] Ignat'ev, N. V.; Barthen, P.; Kucheryna, A.; Willner, H.; Sartori, P. Molecules 2012, 17(5), 5319.
[7] Zheng, D.-Q.; Jing, Y.; Zheng, B.-Y.; Ye, Y.-F.; Xu, S.; Tian, W.-S.; Ma, H.-Y.; Ding, K. Tetrahedron 2016, 72(17), 2164.
[8] (a) Padmapriya, A. A.; Just, G.; Lewis, N. G. Synth. Commun. 1985, 15(12), 1057.
(b) Trujillo, J. I.; Gopalan, A. S. Tetrahedron Lett. 1993, 34(46), 7355.
(c) Yoshino, T.; Togo, H. Synlett 2005.
[9] Ohme, R.; Schmitz, E. Justus Liebigs Ann. Chem. 1968, 716, 207.
[10] Anderson, G. L.; Harruna, I. Synth. Commun. 1987, 17(1), 111.
/
| 〈 |
|
〉 |