

Chinese Journal of Organic Chemistry >
Synthesis of Imidazolium Precursors for the Hydroxyl-Group-Modified N-Heterocyclic Carbenes and Applications of the in situ Generated Carbene Ligands in Suzuki-Miyaura and Sonogashira Coupling Reactions
Received date: 2016-10-22
Revised date: 2016-12-06
Online published: 2017-02-08
Supported by
Project supported by the Basic Research Project of Shanxi Province of China (No. 2015011029), the Undergraduate Innovative Experiment Program of Shanxi Normal University (No. SD2014CXXM-36) and the Shanxi Province Education Innovation Project for Postgraduate (No. 2015BY38).
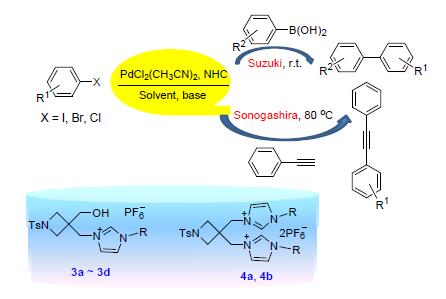
Four imidazolium precursors for the N-heterocyclic carbenes (NHCs) with a hydroxyl functionalized four-mem-bered heterocyclic side arm were synthesized and characterized by IR, XRD and NMR spectroscopies. The corresponding NHC ligands thus generated in situ from these imidazolium precursors in the presence of base such as KOH, together with PdCl2(CH3CN)2, exhibited excellent catalytic activity in Suzuki-Miyaura cross-coupling reactions for the synthesis of a range of biaryl compounds. The reactions could be conducted in the mixed solvent of tert-butyl alcohol/water (V:V=1:1) at room temperature with the advantages of mild conditions, high efficiency as well as environmental friendliness. In addition, although in minor amount, the mixed catalyst system containing imidazolium salts, PdCl2(CH3CN)2 (0.1%) and CuI (1%) exhibited excellent catalytic activity in Sonogashira coupling reaction. In particular, the combination system of PdCl2/imidazolium salt 3b has been shown to be higher catalytically active for both coupling reactions.
Bai Yali , Li Xiaowei , Xiao Xuedong , Liu Jiaqi , Yang Junjuan , Wang Junwen . Synthesis of Imidazolium Precursors for the Hydroxyl-Group-Modified N-Heterocyclic Carbenes and Applications of the in situ Generated Carbene Ligands in Suzuki-Miyaura and Sonogashira Coupling Reactions[J]. Chinese Journal of Organic Chemistry, 2017 , 37(5) : 1258 -1265 . DOI: 10.6023/cjoc201610039
[1] Arduengo, A. J.; Harlow, R. L.; Kline, M. J. Am. Chem. Soc. 1991, 113, 361.
[2] Qu, M. N.; He, J. M. Chin. J. Org. Chem. 2011, 31, 1388 (in Chinese). (屈孟男, 何金梅, 有机化学, 2011, 31, 1388.)
[3] Tang, Y.; Yang, F. F.; Nie, S. P.; Wang, L.; Luo, Z. B.; Lu, H. F. Chin. J. Org. Chem. 2015, 35, 705 (in Chinese). (唐演, 杨飞飞, 聂士鹏, 王林, 罗治斌, 陆鸿飞, 有机化学, 2015, 35, 705.)
[4] Zhang, D.; Zi, G. F. Chem. Soc. Rev. 2015, 44, 1898.
[5] Barroso, S.; de Aguiar, S. R. M. M.; Munhá, R. F.; Martins, A. M. J. Organomet. Chem. 2014, 760, 60.
[6] Zhao, N.; Hou, G. H.; Deng, X. B.; Zi, G. F.; Walter, M. D. Dalton Trans. 2014, 43, 8261.
[7] Hameury, S.; de Frémont, P.; Breuil, P. A. R.; Olivier-Bourbigou, H.; Braunstein, P. Inorg. Chem., 2014, 53, 5189.
[8] Ohmiya, H.; Zhang, H.; Shibata, S.; Harada, A.; Sawamura, M. Angew. Chem. 2016, 128, 4855.
[9] (a)Wang, N. X. Chin. J. Org. Chem. 2011, 31, 1319 (in Chinese). (王乃兴, 有机化学, 2011, 31, 1319.) (b)Maluenda, I.; Navarro, O. Molecules 2015, 20, 7528.
[10] Fortman, G. C.; Nolan, S. P. Chem. Soc. Rev. 2011, 40, 5151.
[11] Levin, E.; Ivry, E.; Diesendruck, C. E.; Lemcoff, N. G. Chem. Rev. 2015, 115, 4607.
[12] Schmid, T. E.; Jones, D. C.; Songis, O.; Diebolt, O.; Furst, M. R. L.; Slawin, A. M. Z.; Cazin, C. S. J. Dalton Trans. 2013, 42, 7345.
[13] Neumann, K. T.; Laursen, S. R.; Lindhardt, A. T.; Bang-Andersen, B.; Skrydstrup, T. Org. Lett. 2014, 16, 2216.
[14] Caddick, S.; Cloke, F. G. N.; Clentsmith, G. K. B.; Hitchcock, P. B.; McKerrecher, D.; Titcomb, L. R.; Williams, M. R. V. J. Organomet. Chem. 2001, 617, 635.
[15] Burkhard, J. A.; Wagner, B.; Fischer, H.; Schuler, F.; Müller, K.; Carreira, E. M. Angew. Chem., Int. Ed. 2010, 49, 3524.
[16] Burkhard, J. A.; Guérot, C.; Knust, H.; Rogers-Evans, M.; Carreira, E. M. Org. Lett. 2010, 12, 1944.
[17] Burkhard, J.; Carreira, E. M. Org. Lett. 2008, 10, 3525.
[18] Wuitschik, G.; Rogers-Evans, M.; Buckl, A.; Bernasconi, M.; Märki, M.; Godel, T.; Fischer, H.; Wagner, B.; Parrilla, I.; Schuler, F.; Schneider, J.; Alker, A.; Schweizer, W. B.; Müller, K.; Carreira, E. M. Angew. Chem. 2008, 120, 4588.
[19] Wang, J. W.; Li, Q. S.; Xu, F. B.; Song, H. B.; Zhang, Z. Z. Eur. J. Org. Chem. 2006, 1310.
[20] Yu, L.; Han, Z. Mater. Lett. 2016, 184, 312.
[21] Al-Amin, M.; Akimoto, M.; Tameno, T.; Ohki, Y.; Takahashi, N.; Hoshiya, N.; Shuto, S.; Arisawa, M. Green Chem. 2013, 15, 1142.
[22] Ma, H. C.; Cao, W.; Bao, Z. K.; Lei, Z. Q. Catal. Sci. Technol. 2012, 2, 2291.
[23] Zhao, X. H.; Zhao, Y. Y.; Zhang, J.; Li, X. Appl. Organomet. Chem. 2015, 29, 840.
[24] Yadav, R. R.; Vishwakarma, R. A.; Bharate, S. B. Tetrahedron Lett. 2012, 53, 5958.
[25] Hassine, A.; Sebti, S.; Solhy, A.; Zahouily, M.; Len, C.; Hedhili, M. N.; Fihri, A. Appl. Catal., A 2013, 450, 13.
[26] Siamaki, A. R.; Lin, Y.; Woodberry, K.; Connell, J. W.; Gupton, B. F. J. Mater. Chem. A 2013, 1, 12909.
[27] Iranpoor, N.; Firouzabadi, H.; Motevalli, S.; Rajabi, K. Aust. J. Chem. 2015, 68, 926.
[28] Zhang, G. P.; Li, P. H. Chin. J. Org. Chem. 2010, 30, 117 (in Chinese). (张国平, 李品华, 有机化学, 2010, 30, 117.)
[29] Elumalai, V.; Bjørsvik, H. R. Tetrahedron Lett. 2016, 57, 1224.
[30] Wang, X.; Wang, Z. H.; Wu, Y.; Luo, Y. L.; Zhang, G. F.; Jian, Y. J.; Sun, H. M.; Zhang, W. Q.; Gao, Z. W. Appl. Organomet. Chem. 2016, 30, 831.
[31] Sagadevan, A.; Hwang, K. C. Adv. Synth. Catal. 2012, 354, 3421.
/
| 〈 |
|
〉 |