

Chinese Journal of Organic Chemistry >
Synthesis and Bioactivities of Novel Pyrazole Oxime Ester Derivatives Containing Pyridyl Moiety
Received date: 2017-01-20
Revised date: 2017-02-21
Online published: 2017-02-27
Supported by
Project supported by the National Natural Science Foundation of China (No. 21372135), the Research Foundation of the Six People Peak of Jiangsu Province (No. 2013-SWYY-013), and the Science and Technology Project Fund of Nantong City (Nos. CP12013002, MS22015020).
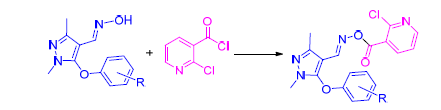
In order to explore novel pyrazole derivatives with good biological activities, a series of novel pyrazole oxime ester compounds containing pyridyl moiety were designed and synthesized according to the method of active substructure combination. The structures of the target compounds were determined by 1H NMR, 13C NMR and elemental analysis. Preliminary bioassay data indicated that some of the title compounds showed certain insecticidal activities. At a concentration of 500 μg/mL, seven compounds exhibited insecticidal activity against Oriental armyworm with 50%~90%, and six compounds exhibited insecticidal activity against Aphis medicaginis with 50%~90%. When the dosage was lowered to 100 μg/mL, 1,3-dimethyl-5-(4-chlorophenoxy)pyrazole-4-formyl-O-(2-chloropyridin-3-formyl)oxime (5f) and 1,3-dimethyl-5-(4-methylphenoxy)pyra-zole-4-formyl-O-(2-chloropyridin-3-formyl)oxime (5j) were still active against Aphis medicaginis with inhibitory values of 50% and 50%, respectively. Insecticidal activities against Nilaparvata lugens of 1,3-dimethyl-5-(3-fluorophenoxy)pyrazole-4-formyl-O-(2-chloropyridin-3-formyl)oxime (5b) and 5f were both 100% at 500 μg/mL. Additionally, 1,3-dimethyl-5-(4-fluorophenoxy)pyrazole-4-formyl-O-(2-chloropyridin-3-formyl)oxime (5c), 1,3-dimethyl-5-(3-chlorophenoxy)-pyrazole-4-for-myl-O-(2-chloropyridin-3-formyl)oxime (5e), 1,3-dimethyl-5-(4-trifluoromethoxyphenoxy)pyrazole-4-formyl-O-(2-chloro-pyridin-3-formyl)oxime (5i) and 5j displayed good anti-tumor activity against HepG2 cells with IC50 values of 2.6, 4.6, 1.8和1.1 μmol/L, respectively.
Key words: pyridine; pyrazole; synthesis; biological activity
Dai Hong , Chen Jia , Hong Yu , Yuan Binying , Fan Chongguang , Ma Ruiyuan , Liang Zhipeng , Shi Jian . Synthesis and Bioactivities of Novel Pyrazole Oxime Ester Derivatives Containing Pyridyl Moiety[J]. Chinese Journal of Organic Chemistry, 2017 , 37(6) : 1542 -1547 . DOI: 10.6023/cjoc201701042
[1] Li, Y.; Zhang, H. Q.; Liu, J.; Yang, X. P.; Liu, Z. J. J. Agric. Food Chem. 2006, 54, 3636.
[2] Dai, H.; Li, Y. Q.; Du, D.; Qin, X.; Zhang, X.; Yu, H. B.; Fang, J. X. J. Agric. Food Chem. 2008, 56, 10805.
[3] Hamaguchi, H.; Kajihara, O.; Katoh, M. J. Pestic. Sci. 1995, 20, 173.
[4] Motoba, K.; Nishizawa, H.; Suzuki, T.; Hamaguchi, H.; Uchida, M.; Funayama, S. Pestic. Biochem. Physiol. 2000, 67, 73.
[5] Park, H. J.; Lee, K.; Park, S. J.; Ahn, B.; Lee, J. C.; Cho, H. Y.; Lee, K. I. Bioorg. Med. Chem. Lett. 2005, 15, 3307.
[6] Hamaguchi, H.; Kajihara, O.; Katoh, M. J. Pestic. Sci. 1995, 20, 173.
[7] Swanson, M. B.; Ivancic, W. A.; Saxena, A. M.; Allton, J. D.; O'Brien, G. K.; Suzuki, T.; Nishizawa, H.; Nokata, M. J. Agric. Food Chem. 1995, 43, 513.
[8] Lahm, G. P., Selby, T. P.; Freudenberger, J. H.; Stevenson, T. M.; Myers, B. J.; Seburyamo, G.; Smith, B. K; Flexner, L.; Clark, C. E.; Cordova, D. Bioorg. Med. Chem. Lett. 2005, 15, 4898.
[9] Penning, T. D.; Talley, J. J.; Bertenshaw, S. R.; Carter, J. S.; Collins, P. W.; Docter, S.; Graneto, M. J.; Lee, L. F.; Malecha, J. W.; Miyashiro, J. M.; Rogers, R. S.; Rogier, D. J.; Yu, S. S.; Anderson, G. D.; Burton, E. G.; Cogburn, J. N.; Gregory, S. A.; Koboldt, C. M.; Perkins, W. E. Seibert, K.; Veenhuizen, A. W.; Zhang, Y. Y.; Isakson, P. C. J. Med. Chem. 1997, 40, 1347.
[10] Teng, M.; Zhu, J. J.; Johnson, M. D.; Chen, P.; Kornmann, J.; Chen, E. T.; Blasina, A.; Register, J.; Anderes, K.; Rogers, C.; Deng, Y. L.; Ninkovic, S.; Grant, S.; Hu, Q. Y.; Lundgren, K.; Peng, Z. W.; Kania, R. S. J. Med. Chem. 2007, 50, 5253.
[11] Ouyang, G. P.; Cai, X. J.; Chen, Z.; Song, B. A.; Bhadury, P. S.; Yang, S.; Jin, L. H.; Xue, W.; Hu, D. Y.; Zeng, S. J. Agric. Food Chem. 2008, 56, 60.
[12] Dai, H.; Shi, L.; Zhang, H. J.; Li, Y. Q.; Fang, J. X.; Shi, Y. J. Chin. J. Org. Chem. 2012, 32, 1060 (in Chinese). (戴红, 施磊, 张海军, 李永强, 方建新, 石玉军, 有机化学, 2012, 32, 1060.)
[13] Wang, X.; Wang, C. Q.; Fu, C. R.; Zou, X. M. Chin. J. Org. Chem. 2015, 35, 92 (in Chinese). (王鑫, 王朝强, 傅翠蓉, 邹小毛, 有机化学, 2015, 35, 92.)
[14] Dai, H.; Zhuang. H. Y.; Shi, L.; Li, G.; Zhang, H. J.; Fang, Y.; Dai, B. J. Chin. J. Org. Chem. 2015, 35, 2399 (in Chinese). (戴红, 庄辉阳, 施磊, 李刚, 张海军, 方源, 戴宝江, 有机化学, 2015, 35, 2399.)
[15] Tian, Z. Z.; Shao, X. S.; Li, Z.; Qian, X. H.; Huang, Q. C. J. Agric. Food Chem. 2007, 55, 2288.
[16] Lu, S. Y.; Shao, X. S.; Li, Z.; Xu, Z. P.; Zhao, S. S.; Wu, Y. L.; Xu, X. Y. J. Agric. Food Chem. 2012, 60, 322.
[17] Selby, T. P.; Lahm, G. P.; Stevenson, T. M.; Hughes, K. A.; Cordova, D.; Annan, I. B.; Barry, J. D.; Benner, E. A.; Currie, M. J.; Pahutski, T. F. Bioorg. Med. Chem. Lett. 2013, 23, 6341.
[18] Song, B. A.; Liu, X. H.; Yang, S.; Hu, D. Y.; Jin, L. H.; Zhang, Y. T. Chin. J. Org. Chem. 2005, 25, 507 (in Chinese). (宋宝安, 刘新华, 杨松, 胡德禹, 金林红, 张玉涛, 有机化学, 2005, 25, 507.)
[19] Ouyang, G. P.; Chen, Z.; Cai, X. J.; Song, B. A.; Bhadury, P. S.; Yang, S.; Jin, L. H.; Xue, W.; Hu, D. Y., Zeng, S. Bioorg. Med. Chem. 2008, 16, 9699.
[20] Shi, Y. J.; Wang, S. L.; He, H. B.; Li, Y.; Li, Y.; Fang, Y.; Dai, H. Chin. J. Org. Chem. 2015, 35, 1785 (in Chinese). (石玉军, 王森林, 何海兵, 李钰, 李阳, 方源, 戴红, 有机化学, 2015, 35, 1785.)
[21] Dai, H.; Li, H.; Jin. Z. C.; Liu, W. Y.; Xiao, Y.; He, H. B.; Wang, Q. M.; Shi, Y. J. Chin. J. Org. Chem. 2016, 36, 185 (in Chinese). (戴红, 李宏, 金智超, 刘文永, 肖瑶, 何海兵, 汪清民, 石玉军, 有机化学, 2016, 36, 185.)
[22] Song, H. J.; Liu, Y. X.; Xiong, L. X.; Li, Y. Q.; Yang, N.; Wang, Q. M. J. Agric. Food Chem. 2013, 61, 8730.
[23] Liu, X. H.; Cui, P.; Song, B. A.; Bhadury, P. S.; Zhu, H. L.; Wang, S. F. Bioorg. Med. Chem. 2008, 16, 4075.
[24] Park, M. S.; Park, H. J.; Park, K. H.; Lee, K. I. Synth. Commun. 2004, 34, 1541.
[25] Ma, J. A.; Huang, R. Q.; Feng, L.; Song, J.; Qiu, D. W. Chem. Res. Chin. Univ. 2003, 19, 297.
[26] Dai, H.; Xiao, Y. S.; Li, Z.; Xu, X. Y.; Qian, X. H. Chin. Chem. Lett. 2014, 25, 1014.
[27] Liu, J. C.; Liu, Y. J.; He, H. W. Chin. J. Org. Chem. 2015, 35, 462 (in Chinese). (刘建超, 刘勇军, 贺红武, 有机化学, 2015, 35, 462.)
[28] Song, B. A.; Yang, S.; Zhong, H. M.; Jin, L. H.; Hu, D. Y.; Liu, G. J. Fluorine Chem. 2015, 126, 87.
/
| 〈 |
|
〉 |