

Chinese Journal of Organic Chemistry >
One-Pot Synthesis and Optical Properties of 2,5-Diphenylthiophene Derivatives
Received date: 2016-12-15
Revised date: 2017-02-22
Online published: 2017-03-08
Supported by
Project supported by the Natural Science Foundation of the Jiangsu Higher Education Institutions (No.14JKD150009) and the Excellent Specialties Program Development of Jiangsu Higher Education Institutions.
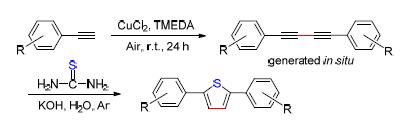
The paper describes a convenient and facile methodology for the synthesis of 2,5-diphenylthiophene derivatives. The environmentally friendly synthetic approach is supported by a one-pot tandem reaction process. All of the target products was confirmed by 1H NMR, 13C NMR and HRMS. On this basis, UV and fluorescence properties of the synthesized compounds were further explored. The experimental results showed that the UV maximum absorption wavelengths of the compounds are between 292 and 341 nm. The fluorescence spectra showed that these compounds have good fluorescence. The fluorescence emission wavelengths measured in methanol are between 386 and 454.5 nm, and the fluorescence emission wavelengths measured in dichloromethane are between 390 and 412 nm. The increase of conjugation system led to the red shift of fluorescence.
Qian Cunwei , Zang Shiyu , Zhou Qian , Wang Dong , Li Wanxin , Wang Maoyuan . One-Pot Synthesis and Optical Properties of 2,5-Diphenylthiophene Derivatives[J]. Chinese Journal of Organic Chemistry, 2017 , 37(7) : 1781 -1786 . DOI: 10.6023/cjoc201612045
[1] For selected review, see:(a) Wu, X. F.; Neumann, H.; Beller, M. Chem. Rev. 2013, 113, 1.
(b) Zeni, G.; Larock, R. C. Chem. Rev. 2006, 106, 4644.
(c) Lipshutz, B. H. Chem. Rev. 1986, 86, 795.
(d) Lu, H.; Liu, G. T. Planta Med. 1992, 58, 311.
(e) Navarro, E.; Alonso, S. J.; Trujillo, J.; Jorge, E.; Pérez, C. J. Nat. Prod. 2001, 64, 134.
(f) Cacchi, S.; Fabrizi, G.; Goggiamani, A. Org. Biomol. Chem. 2011, 9, 641.
(g) Flynn, B. L.; Hamel, E.; Jung, M. K. J. Med. Chem. 2002, 45, 2670.
(h) Palkowitz, A. D.; Glasebrook, A. L.; Thrasher, K. J.; Hauser, K. L.; Short, L. L.; Philips, D. L.; Muehl, B. S.; Sato, M.; Shetler, P. K.; Cullinan, G. J.; Pell, T. R.; Bryant, H. U. J. Med. Chem. 1997,40, 1407.
(i) Tsuji, H.; Cantagrel, G.; Ueda, Y.; Chen, T.; Wan, L. J.; Nakamura, E. Chem. Asian J. 2013, 8, 2377.
[2] Joule, J. A.; Mills, K. Heterocyclic Chemistry, Trans. by You, Y.-C.; Gao, D.-B. Science Press, Beijing, 2004, p. 324(in Chinese). (J. A. 焦耳, K. 米尔斯, 杂环化学, 由业诚, 方大彬译, 科学出版社, 北京, 2004, p. 324.)
[3] (a) Snégaroff, K.; Komagawa, S.; Chevallier, F.; Gros, P. C.; Golhen, S.; Roisnel, T.; Uchiyama, M.; Mongin, F. Chem.-Eur. J. 2010, 16, 8191.
(b) Kel'in, A. V.; Gevorgyan, V. J. Org. Chem. 2002, 67, 95.
(c) Rao, H. S. P.; Jothilingam, S. J. Org. Chem. 2003, 68, 5392.
(d) Aponick, A.; Li, C. Y.; Malinge, J.; Marques, E. F. Org. Lett. 2009, 11, 4624.
(e) Egi, M.; Azechi, K.; Akai, S. Org. Lett. 2009, 11, 5002.
(f) Dheur, J.; Sauthier, M.; Castanet, Y.; Mortreux, A. Adv. Synth. Catal. 2010, 352, 557.
[4] (a) Sun, H.; Wu, X.; Hua, R. Tetrahedron Lett. 2011, 52, 4408.
(b) Singha, R.; Nandi, S.; Ray, J. K. Tetrahedron Lett. 2012, 53, 6531.
(c) Pridmore, S. J.; Slatford, P. A.; Williams, J. M. J. Tetrahedron Lett. 2007, 48, 5111.
(d) Kramer, S.; Madsen, J. L. H.; Rottl€ander, M.; Skrydstrup, T. Org. Lett. 2010, 12, 2758.
(e) Nun, P.; Dupuy, S.; Gaillard, S.; Poater, A.; Cavallod, L.; Nolan, S. P. Catal. Sci. Technol. 2011, 1, 58.
(f) Jiang, H.; Zeng, W.; Li, Y.; Wu, W.; Huang, L.; Fu, W. J. Org. Chem. 2012, 77, 5179.
(g) Zheng, Q.; Hua, R.; Yin, T. Curr. Org. Synth. 2013, 10, 161.
(h) Beny, J.-P.; Dhawan, S. N.; Kagan, J.; Sundlass, S. J. Org. Chem. 1982, 47, 2201.
(i) Pridmore, S. J.; Slatford, P. A.; Daniel, A.; Whittlesey, M. K.; Williams, J. M. J. Tetrahedron Lett. 2007, 48, 5115.
(j) Lavallo, V.; Frey, G. D.; Donnadieu, B.; Soleilhavoup, M.; Bertrand, G. Angew. Chem., Int. Ed. 2008, 47, 5224.
(k) Zheng, Q.; Hua, R. Tetrahedron Lett. 2010, 51, 4512.
(l) Mandadapu, A. K.; Sharma, S. K.; Gupta, S.; Krishna, D. G. V.; Kundu, B. Org. Lett. 2011, 13, 3162.
(m) Mandadapu, A. K.; Dathi, M. D.; Arigela, R. K.; Kundu, B. Tetrahedron 2012, 68, 8207.
(n)Wang, L.; Yu, X.; Feng, X.; Bao, M. Org. Lett. 2012, 14, 2418.
(o) Zheng, Q.; Hua, R.; Yin, T. Curr. Org. Synth. 2013, 10, 161.
(p) Jiang, H.; Zeng, W.; Li, Y.; Wu, W.; Huang, L.; Fu, W. J. Org. Chem. 2012, 77, 5179.
[5] (a) Zheng, Q.; Hua, R.; Jiang, J.; Zhang, L. Tetrahedron 2014, 70, 8252.
(b) Zhang, G.; Yi, H.; Chen, H.; Bian, C.; Liu, C.; Lei, A. Org. Lett. 2014, 16, 6156.
(c) Tang, J.; Zhao, X. RSC Adv. 2012, 2, 5488.
[6] (a) Li, D.; Yin, K.; Li, J.; Jia, X. Tetrahedron Lett. 2008, 49, 5918.
(b) Li, L.; Wang, J.; Zhang, G.; Liu, Q. Tetrahedron Lett. 2009, 50, 4033.
[7] Urselmann, D.; Antovic, D.; Müller T. Beilstein J. Org. Chem. 2011, 7, 1499.
[8] Irudayanathan, F. M.; Raja, G. C. E.; Lee, S. Tetrahedron 2015, 71, 4418.
/
| 〈 |
|
〉 |