

Chinese Journal of Organic Chemistry >
N-Heterocyclic Carbene-Palladium(Ⅱ) Complexes with Acridine Ligand:Synthesis, Characterization and Catalytic Applications
Received date: 2017-01-19
Revised date: 2017-03-02
Online published: 2017-03-08
Supported by
Project supported by the National Natural Sciences Foundation of China (Nos.U1404205,21572126 and 21202095),the Program for Science&Technology Innovation Talents in Universities of Henan Province (No.14HASTIT016) and the Key Scientific and Technological Project of Henan Province (No.152102410056).
Nine new heterozygous isatin-quinazoline compounds were synthesized from cheap and easily available ortho nitrobenzaldehyde as the starting material.The chemical structures of the synthesized compounds were characterized by NMR,IR and HRMS.The structure of (E)-3-(((E)-(5-(4-((3-ethynylphenyl) amino) quinazolin-6-yl) furan-2-yl) methylene) hydrazono)-indolin-2-one (4a) was further determined by crystallization and X-ray diffraction,and the data revealed that its cis-trans isomerism was (E,E).The antitumor activity of these new compounds was evaluated in vitro by methyl thiazolyl tetrazolium (MTT) assay in human colorectal carcinoma cells SW480,human non-small cell lung cancer cells A549 and NCI- H1975,and human epidermoid squamous carcinoma cells A431.The preliminary data demonstrated that most of the synthetic compounds had moderate to potent inhibitory activity against these four tumor cell lines.In particular,compound 4a had highly potent inhibitory activity on proliferation of four cell lines,and the activity was more potent than positive lapatinib.
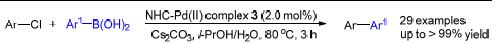
Key words: N-heterocyclic carbene; palladium; acridine; Suzuki-Miyaura cross-coupling
Wang Tao , Xu Kai , Meng Tuanjie , Zhang An'an , Wang Hongyu , Shen Sisi , Liu Lantao . N-Heterocyclic Carbene-Palladium(Ⅱ) Complexes with Acridine Ligand:Synthesis, Characterization and Catalytic Applications[J]. Chinese Journal of Organic Chemistry, 2017 , 37(7) : 1794 -1799 . DOI: 10.6023/cjoc201701039
[1] Miyaura, N.; Suzuki, A. Chem. Rev. 1995, 95, 2457.
[2] Suzuki, A. J. Organomet. Chem. 1999, 576, 147.
[3] Corbet, J.-P.; Mignani, G. Chem. Rev. 2006, 106, 2651.
[4] Jiang, L.; Li, Z.; Zhao, D. Chin. J. Org. Chem. 2010, 30, 200(in Chinese). (姜岚, 李争宁, 赵德峰, 有机化学, 2010, 30, 200.)
[5] Zhang, G.; Zhang, W.; Luan, Y.; Han, X.; Ding, C. Chin. J. Chem. 2015, 33, 705.
[6] Liu, C.; Liu, G.; Zhao, H. Chin. J. Chem. 2016, 34, 1048.
[7] Li, Q.; Ding, Y.; Zhang, G.; Zhang, Z.; Mo, S. Chin. J. Org. Chem. 2016, 36, 83(in Chinese). (李清寒, 丁勇, 张刚, 张震, 莫松, 有机化学, 2016, 36, 83.)
[8] Yuan, D.; Zhang, Q.; Liao, S.; Xiong, W.; Yuan, L.; Cai, Q.; Yang, M.; Li, X.; Jiang, Y.; Liu, Y.; Li, P.; Xu, Z.; Sun, P.; Geng, H. Chin. J. Org. Chem. 2015, 35, 961(in Chinese). (袁定重, 张庆华, 廖世军, 熊文文, 元利刚, 蔡奇胜, 杨梦梅, 李雄, 蒋烨佳, 刘妍, 李萍, 徐贞帅, 孙盼盼, 耿会玲, 有机化学, 2015, 35, 961.)
[9] Gu, N.; Liu, Y.; Liu, P.; Ma, X.; Liu, Y.; Dai, B. Chin. J. Chem. 2015, 33, 1189.
[10] Buchwald, S. L.; Wolfe, J. P.; Old, D. W. J. Am. Chem. Soc. 1998, 120, 9722.
[11] Littke, A. F.; Fu, G. C. Angew. Chem., Int. Ed. 1998, 37, 3387.
[12] Sau, S. C.; Santra, S.; Sen, T. K.; Mandal, S. K.; Koley, D. Chem. Commun. 2012, 48, 555.
[13] Yuan, B.; Pan, Y.; Li, Y.; Yin, B.; Jiang, H. Angew. Chem., Int. Ed. 2010, 49, 4054.
[14] Diez-Gonzalez, S.; Marion, N.; Nolan, S. P. Chem. Rev. 2009, 109, 3612.
[15] Droge, T.; Glorius, F. Angew. Chem., Int. Ed. 2010, 49, 6940.
[16] Fortman, G. C.; Nolan, S. P. Chem. Soc. Rev. 2011, 40, 5151.
[17] Budagumpi, S.; Haque, R. A.; Salman, A. W. Coord. Chem. Rev. 2012, 256, 1787.
[18] Tang, Y.; Yang, F.; Nie, S.; Wang, L.; Luo, Z.; Lu, H. Chin. J. Org. Chem. 2015, 35, 705(in Chinese). (唐演, 杨飞飞, 聂士鹏, 王林, 罗治斌, 陆鸿飞, 有机化学, 2015, 35, 705.)
[19] Tu, T.; Sun, Z.; Fang, W.; Xu, M.; Zhou, Y. Org. Lett. 2012, 14, 4250.
[20] Teci, M.; Brenner, E.; Matt, D.; Toupet, L. Eur. J. Inorg. Chem. 2013, 2841.
[21] Rajabi, F.; Thiel, W. R. Adv. Synth. Catal. 2014, 356, 1873.
[22] Dunsford, J. J.; Cavell, K. J. Organometallics 2014, 33, 2902.
[23] Lv, H.; Zhu, L.; Tang, Y.-Q.; Lu, J.-M. Appl. Organomet. Chem. 2014, 28, 27.
[24] Zhang, Y.; Feng, M.-T.; Lu, J.-M. Org. Biomol. Chem. 2013, 11, 2266.
[25] Wang, Z.-Y.; Chen, G.-Q.; Shao, L.-X. J. Org. Chem. 2012, 77, 6608.
[26] Yin, H.-Y.; Liu, M.-Y.; Shao, L.-X. Org. Lett. 2013, 15, 6042.
[27] Gu, Z.-S.; Chen, W.-X.; Shao, L.-X. J. Org. Chem. 2014, 79, 5806.
[28] Shen, X.-B.; Zhang, Y.; Chen, W.-X.; Xiao, Z.-K.; Hu, T.-T.; Shao, L.-X. Org. Lett. 2014, 16, 1984.
[29] Micksch, M.; Tenne, M.; Strassner, T. Organometallics 2014, 33, 3966.
[30] Shen, A.; Ni, C.; Cao, Y.-C.; Zhou, H.; Song, G.-H.; Ye, X.-F. Tetrahedron Lett. 2014, 55, 3278.
[31] Yang, J.; Wang, L. Dalton Trans. 2012, 41, 12031.
[32] Yang, J.; Li, P.; Zhang, Y.; Wang, L. J. Organomet. Chem. 2014, 766, 73.
[33] Yang, J.; Li, P.; Zhang, Y.; Wang, L. Dalton Trans. 20014, 43, 7166.
[34] Wang, T.; Xie, H.; Liu, L.; Zhao, W.-X. J. Organomet. Chem. 2016, 804, 73.
[35] Ouyang, K.; Xi, Z. Acta Chim. Sinica 2013, 71, 13(in Chinese). (欧阳昆冰, 席振峰, 化学学报, 2013, 71, 13.)
[36] Sheldrick, G. M. SHELXS-97, Program for Crystal Structure Solution, University of Göttingen, Göttingen, Germany, 1997.
[37] Sheldrick, G. M. SHELXL-97, Program for Crystal Structure Refinement, University of Göttingen, Göttingen, Germany, 1997.
/
| 〈 |
|
〉 |