

Chinese Journal of Organic Chemistry >
Tetrabutylammonium Iodide/t-Butylhydroperoxide Catalytic/Oxidative Synthesis of Thiazolylidene Derivatives
Received date: 2017-01-22
Revised date: 2017-03-05
Online published: 2017-03-08
Supported by
Project supported by the National Natural Science Foundation of China (No.21372137).
Two novel N-heterocyclic carbene-palladium (II) complexes were conveniently synthesized through one-pot reactions of imidazolium salts,palladium chloride and acridine.The new complexes have been fully characterized by 1H NMR,13C NMR,elemental analysis,and X-ray single-crystal diffraction.Moreover,the obtained palladium (II) complexes were the effective catalyst precursors for the Suzuki-Miyaura coupling of aryl as well as benzyl chlorides with arylboronic acids.Under the optimal conditions,all reactions proceeded successfully to give the desired products in good to almost quantitative yields.
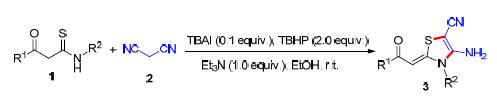
Key words: thiazolylidenes; β-ketothioamides; TBAI/TBHP; catalysis
Yu Le , Liu Ruijuan , Li Ming . Tetrabutylammonium Iodide/t-Butylhydroperoxide Catalytic/Oxidative Synthesis of Thiazolylidene Derivatives[J]. Chinese Journal of Organic Chemistry, 2017 , 37(7) : 1800 -1807 . DOI: 10.6023/cjoc201701045
[1] Kashyap, S. J.; Garg, V. K.; Sharma, P. K.; Kumar, N.; Dudhe, R.; Gupta, J. K. Med. Chem. Res. 2012, 21(8), 2123.
[2] (a) Meneely, K. M.; Ronnebaum, T. A.; Riley, A. P.; Prisinzano, T. E.; Lamb, A. L. Biochemistry 2016, 55, 5423.
(b) Boyce, R. J.; Pattende, G. Tetrahedron 1995, 51(26), 7313.
[3] Carroll, W. A.; Dart, M. J.; Kolasa, T.; Meyer, M. D.; Patel, M. V.; Wang, X.-Q. WO 2008063781, 2008[Chem. Abstr. 2008, 639601]
[4] Takamizawa, A.; Harada, H. DE 2458933, 1975[Chem. Abstr. 1975, 514384]
[5] (a) Iravani, N.; Keshavarz, M.; Monfareda, M.; Hosseini, F. J. Chin. Chem. Soc. 2014, 61, 357.
(b) Yavari, I.; Hosseinpour, R.; Pashazadeh, R.; Ghanbari, E.; Skoulika, S. Tetrahedron 2013, 69, 2462.
(c) Alya, A. A.; Ishaka, E. A.; Brown, A. B. J. Sulfur Chem. 2014, 35(4), 382.
[6] (a) Wu, X.-F.; Gong, J.-L.; Qi, X.-X. Org. Biomol. Chem. 2014, 12, 5807.
(b) Hao, W.-J.; Du, Y.; Wang, D.; Jiang, B.; Gao, Q.; Tu, S.-J.; Li, G.-G. Org. Lett. 2016, 18, 1884.
(c) Liu, Z.-J.; Zhang, J.; Chen, S.-L.; Shi, E.-B.; Xu, Y.; Wan, X.-B. Angew. Chem., Int. Ed. 2012, 51, 3231.
(d) Zhang, Y.; Hu, G.-B.; Ma, D.-M.; Xu, P.-X.; Gao, Y.-X.; Zhao, Y.-F. Chem. Commun. 2016, 52, 2815.[e] Tang, Y.-C.; Zhang, M.; Li, X.-Q.; Xu, X.-S.; Du, X.-H. Chin. J. Org. Chem. 2015, 35, 875(in Chinese). (唐裕才, 张敏, 李小青, 许响生, 杜晓华, 有机化学, 2015, 35, 875.)
(f) Ma, L.-J.; Wang, X.-P.; Yu, W.; Han, B.. Chem. Commun. 2011, 47, 11333.
(g) Wang, Q.; Xu, Z. Chin. J. Org. Chem. 2013, 33, 2430(in Chinese). (王倩, 徐洲, 有机化学, 2013, 33, 2430.)
(h) Yu, H.; Chen, Y.-G.; Zhang, Y.-H. Chin. J. Chem. 2015, 33(5), 531.
(i) Yu, H.; Zhang, Y.-H. Chin. J. Chem. 2016, 34(4), 359.
[7] (a) Zhang, L.; Dong, J.-H.; Xu, X.-X.; Liu, Q. Chem. Rev. 2016, 116, 287.
(b) Pan, L.; Bi, X.-H.; Liu, Q. Chem. Soc. Rev. 2013, 42, 1251.
(c) Luo, X.-Y.; Ge, L.-S.; An, X.-L.; Jin, J.-H.; Wang, Y.; Sun, P.-P.; Deng, W.-P. J. Org. Chem. 2015, 80, 4611.
(d) Singh, M. S.; Nandi, G. C.; Samai, S. Green Chem. 2012, 14, 447.
(e) Huang, F.; Wu, P.; Wang, L.-D.; Chen, J.-P.; Sun, C.-L.; Yu, Z.-K. Chem. Commun. 2014, 50, 12479.
(f) Wen, L.-R.; He, T.; Lan, M.-C.; Li, M. J. Org. Chem. 2013, 78, 10617.
(g) Nandi, G. C.; Singh, M. S. J. Org. Chem. 2016, 81, 5824.
(h) Yugandar, S.; Konda, S.; Parameshwarappa, G.; Ila, H. J. Org. Chem. 2016, 81, 5606.
[8] Guo, W.-S.; Wen, L-R.; Li, M. Org. Biomol. Chem. 2015, 13, 1942.
[9] (a) Wen, L.-R.; Sun, J.-H.; Li, M.; Sun, E.-T.; Zhang, S.-S. J. Org. Chem. 2008, 73, 1852.
(b) Li, M.; Zuo, Z.-Q.; Wen, L.-R.; Wang, S.-W. J. Comb. Chem. 2008, 10, 436.
(c) Wen, L.-R.; Shi, Y.-J.; Liu, G.-Y.; Li, M. J. Org. Chem. 2012, 77, 4252.
(d) Li, M.; Hou, Y.-L.; Wen L.-R.; Gong, F.-M. J. Org. Chem. 2010, 75, 8522.
(e) Li, M.; Shao, P.; Wang, S.-W.; Kong, W.; Wen, L.-R. J. Org. Chem. 2012, 77, 8956.
[10] (a) Maiboroda, E. I.; Britsun, V. N. Russ. J. Org. Chem. 2008, 44, 1200.
(b) Britsun, V. N. Russ. J. Org. Chem. 2008, 44, 1528.
[11] Wen, L.-R.; Men, L.-B.; He, T.; Ji, G.-J. Li, M. Chem.-Eur. J. 2014, 20, 5028.
[12] (a) Li, M.; Cao, H.; Wang, Y.; Lv, X.-L.; Wen, L.-R. Org. Lett. 2012, 14, 3470.
(b) Li, M.; Kong, X.-J.; Wen, L.-R. J. Org. Chem. 2015, 80, 11999.
(c) Guo, W.-S.; Xin, X.; Zhao, K.-L.; Wen, L.-R.; Li, M. RSC Adv. 2015, 5, 70429.
[13] (a) Qiu, J.-K.; Hao, W.-J. Wang, D.-C.; Wei, P.; Sun, J.; Jiang, B.; Tu, S.-J. Chem. Commun. 2014, 50, 14782.
(b) Zhang, J.; Shao, Y.; Wang, H.-X.; Luo, Q.; Chen, J.-J.; Xu, D.-M.; Wan, X.-B. Org. Lett. 2014, 16, 3312.
(c) Mondal, B.; Sahoo, S. C. Pan, S. C. Eur. J. Org. Chem. 2015, 3135.
[14] (a) Uyanik, M.; Okamoto, H.; Yasui, T.; Ishihara, K. Science 2010, 328, 1376.
(b) Ma, L.-J.; Wang, X.-P.; Yu, W.; Han, B. Chem. Commun. 2011, 47, 11333.
(c) Yuan, Y.-C.; Hou, W.-J.; Zhang-Negrerie, D.; Zhao, K.; Du, Y.-F. Org. Lett. 2014, 16, 5410.
(d) Xu, W.; Nachtsheim, B. J. Org. Lett. 2015, 17, 1585.
/
| 〈 |
|
〉 |