

Chinese Journal of Organic Chemistry >
Synthesis and Antitumor Activities of Novel Bivalent 1-Heterocyclic-β-carbolines Linked by Alkylamino Spacer
Received date: 2017-01-04
Revised date: 2017-03-07
Online published: 2017-03-17
Supported by
Project supported by the Program for Outstanding Youth Science and Technology Innovation Talents Training in Xinjiang Uygur Autonomous Region (No.qn2015jc067).
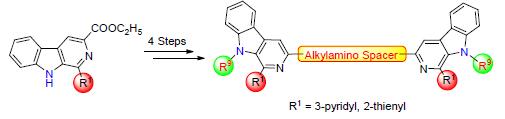
In order to find novel antitumor candidate compounds with high efficiency and low toxicity, a series of 1-heterocyclic substituted bivalent β-carbolines with a spacer of four or five methylene units between the two 3-methylamino group were synthesized, and the chemical structures were characterized by 1H NMR, 13C NMR, and HRMS. The cytotoxic activities of all bivalent β-carbolines were evaluated in vitro against a panel of human tumor cell lines (22RV1, SK-OV-3, MCF-7, LLC, Eca-109, BGC-823, HT-29, HepG-2, A375, and 769-P) and compared with the positive control cisplatin and monovalent β-carbolines. The results demonstrated that compounds 5a~5h exhibited potent cytotoxic activities with IC50 values lower than 10 μmol·L-1. In particular, compounds 5d and 5h, both of which had a spacer of five methylene units, exhibited significant inhibitory activity against 769-P and 22RV1 with IC50 values of 0.8 μmol·L-1 and 0.6 μmol·L-1, respectively.
Guo Liang , Xie Jianwei , Fan Wenxi , Chen Wei , Dai Bin , Ma Qin . Synthesis and Antitumor Activities of Novel Bivalent 1-Heterocyclic-β-carbolines Linked by Alkylamino Spacer[J]. Chinese Journal of Organic Chemistry, 2017 , 37(7) : 1741 -1747 . DOI: 10.6023/cjoc201701005
[1] Michael, C.; Robert, W. W.; Fil, G.; James, M. C.; Steven, A. B.; Kenner, C. R.; Jacqueline, N. C.; Steven, M. P.; Phil, S. J. Med. Chem. 1982, 25, 1081.
[2] Rashid, M. A.; Gustafson, K. R.; Boyd, M. R. J. Nat. Prod. 2001, 64, 1454.
[3] Kuo, P.-C.; Shi, L.-S.; Damu, A. G.; Su, C.-R.; Huang, C.-H.; Ke, C.-H.; Wu, J.-B.; Lin, A.-J.; Bastow, K. F.; Lee, K.-H.; Wu, T.-S. J. Nat. Prod. 2003, 66, 1324.
[4] Srivastava, S. K.; Agarwal, A.; Chauhan, P. M. S.; Agarwal, S. K.; Bhaduri, A. P.; Singh, S. N.; Fatima, N.; Chatterjee, R. K. Bioorg. Med. Chem. 1999, 7, 1223.
[5] Wang, Y.-H.; Tang, J.-G.; Wang, R.-R.; Yang, L.-M.; Dong, Z.-J.; Du, L.; Shen, X.; Liu, J.-K.; Zheng, Y.-T. Biochem. Biophys. Res. Commun. 2007, 355, 1091.
[6] Shankaraiah, N.; Siraj, K. P.; Nekkanti, S.; Srinivasulu, V.; Sharma, P.; Senwar, K. R.; Sathish, M.; Vishnuvardhan, M. V. P. S.; Ramakrishna, S.; Jadala, C.; Nagesh, N.; Kamal, A. Bioorg. Chem. 2015, 59, 130.
[7] Kamal, A.; Sathish, M.; Nayak, V. L.; Srinivasulu, V.; Kavitha, B.; Tangella, Y.; Thummuri, D.; Bagul, C.; Shankaraiah, N.; Nagesh, N. Bioorg. Med. Chem. 2015, 23, 5511.
[8] Figueiredo, P. O.; Perdomo, R. T.; Garcez, F. R.; Matos, M. F. C.; Carvalho, J. E.; Garcez, W. S. Bioorg. Med. Chem. Lett. 2014, 24, 1358.
[9] Li, Y.; Liang, F.-S.; Jiang, W.; Yu, F.-S.; Cao, R.-H.; Ma, Q.-H.; Dai, X.-Y.; Jiang, J.-D.; Wang, Y.-C.; Si, S.-Y. Cancer Biol. Ther. 2007, 6, 1193.
[10] Zhang, J.; Li, Y.; Guo, L.; Cao, R.-H.; Zhao, P.; Jiang, W.; Ma, Q.; Yi, H.; Li, Z.-R.; Jiang, J.-D.; Wu, J.-L.; Wang, Y.-C.; Si, S.-Y. Cancer Biol. Ther. 2009, 8, 2374.
[11] Barsanti, P. A.; Wang, W.; Ni, Z.; Duhl, D.; Brammeier, N.; Martin, E.; Bussiere, D.; Walter, A. O. Bioorg. Med. Chem. Lett. 2010, 20, 157.
[12] Castro, A. C.; Dang, L. C.; Soucy, F.; Grenier, L.; Mazdiyasni, H.; Hottelet, M.; Parent, L.; Pien, C.; Palombella, V.; Adams, J. Bioorg. Med. Chem. Lett. 2003, 13, 2419.
[13] Gaugain, B.; Barbet, J.; Capelle, N.; Roques, B. P.; Le Pecp, J. B.; Le Bret, M. Biochemisry 1978, 17, 5078.
[14] Capelle, N.; Barbet, J.; Dessen, P.; Blanquet, S.; Roques, B. P.; Le Pecq, J. B. Biochemisry 1979, 18, 3354.
[15] Wang, K.-B.; Di, Y.-T.; Bao, Y.; Yuan, C.-M.; Chen, G.; D. Li, H.; Bai, J.; He, H.-P.; Hao, X.-J.; Pei, Y.-H.; Jing, Y.-K.; Li, Z.-L.; Hua, H.-M. Org. Lett. 2014, 16, 4028.
[16] Joshi, A.; Vance, D.; Rai, P.; Thiyagarajan, A.; Kane, R. S. Chem. Eur. J. 2008, 14, 7738.
[17] Brana, M. F.; Castellano, J. M.; Moran, M.; Perez de Vega, M. J.; Perron, D.; Conlon, D.; Bousquet, P. F.; Romerdahl, C. A.; Robinson, S. P. Anticancer Drug Des. 1996, 11, 297
[18] Burns, M. R.; Turner, S. L.; Ziemer, J.; Vean, M. M.; Devens, B.; Carlson, C. L.; Graminski, G. F.; Vanderwerf, S. M.; Weeks, R. S.; Carreon, J. Bioorg. Med. Chem. Lett. 2002, 12, 1263.
[19] Guo, L.; Sun, J.; Fan, W.-X.; Ma, Q. Chin. J. Modern Appl. Pharm. 2012, 29, 385(in Chinese). (郭亮, 孙洁, 范文玺, 马芹, 中国现代应用药学, 2012, 29, 385.)
[20] Guo, L.; Cao, R.-H.; Fan, W.-X.; Ma, Q. Chem. J. Chin. Univ. 2014, 35, 518(in Chinese). (郭亮, 曹日晖, 范文玺, 马芹, 高等学校化学学报, 2014, 35, 518.)
[21] Guo, L.; Fan, W.-X.; Chen, X.-M.; Ma, Q.; Cao, R.-H. Chin. J. Org. Chem. 2013, 33, 332(in Chinese). (郭亮, 范文玺, 陈雪梅, 马芹, 曹日晖, 有机化学, 2013, 33, 332.)
[22] Guo, L.; Fan, W.-X.; Chen, W.; Ma, Q.; Cao, R.-H. J. Chin. Pharm. Sci. 2015, 24, 801.
[23] Zhang, G.-X.; Cao, R.-H.; Guo, L.; Ma, Q.; Fan, W.-X.; Chen, X.-M., Li, J.-R., Shao, G.; Qiu, L.-Q.; Ren, Z.-H. Eur. J. Med. Chem. 2013, 65, 21.
[24] Shi, B.-X.; Cao, R.-H.; Fan, W.-X.; Guo, L.; Ma, Q.; Chen, X.-M.; Zhang, G.-X.; Qiu, L.-Q.; Song, H.-C. Eur. J. Med. Chem. 2013, 60, 10.
[25] Wu, Q.-F.; Bai, Z.-S.; Ma, Q.; Fan, W.-X.; Guo, L.; Zhang, G.-X.; Qiu, L.-Q.; Yu, H.; Shao, G.; Cao, R.-H. Med. Chem. Commun. 2014, 5, 953.
[26] Guo, L.; Cao, R.-H.; Fan, W.-X.; Gan, Z.-Y.; Ma, Q. Chem. J. Chin. Univ. 2016, 37, 1093(in Chinese). (郭亮, 曹日晖, 范文玺, 甘紫云, 马芹, 有机化学, 2016, 37, 1093.)
[27] Chen, J.; Zhang, Y.-K.; Zhan, X.-P.; Liu, Z.-L.; Mao, Z.-M. Chin. J. Org. Chem. 2016, 36, 572(in Chinese).(陈简, 张袁魁, 詹晓平, 刘增路, 毛振民, 有机化学, 2016, 36,572.)
/
| 〈 |
|
〉 |