

Chinese Journal of Organic Chemistry >
Synthesis of 1,4-Dihydropyridine Compounds Catalyzed by Humic Acid
Received date: 2016-12-22
Revised date: 2017-03-13
Online published: 2017-04-10
Supported by
Project supported by the Open Fund of Anhui Collaborative Innovation Center for Advanced Function Composites (No.20160804).
A novel, efficient and straightforward method to synthesize 1,4-dihydropyridine compounds with excellent yields is reported through Hantzsch reaction of aldehydes, ammonium acetate and ethyl acetoacetate or methyl acetoacetate using humic acid as the catalyst. This method has high yield and less pollution. The catalyst is easy to recycle and environment friendly.
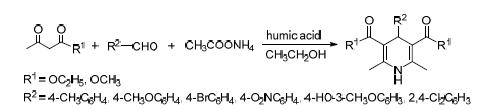
Key words: humic acid; catalysis; 1,4-dihydropyridine
Wei Zhenzhong , Li Jiangfei , Wang Zeyun , Li Pinhua , Wang Yongqiu . Synthesis of 1,4-Dihydropyridine Compounds Catalyzed by Humic Acid[J]. Chinese Journal of Organic Chemistry, 2017 , 37(7) : 1835 -1838 . DOI: 10.6023/cjoc201612055
[1] Goldmann, S.; Stoltefuss, J. Angew. Chem., Int. Ed. 1991, 30, 1559.
[2] Lu, L.-L.; Xu, H.; Zhou, P.; Yu, F.-C. Chin. J. Org. Chem. 2016, 36, 2858(in Chinese). (鲁玲玲, 许辉, 周攀, 余富朝, 有机化学, 2016, 36, 2858.)
[3] Soo-Jeong, C.; Joong-Heui, C.; Isak, I. Eur. J. Med. Chem. 2010, 45, 2578.
[4] Hilgroth, A.; Lilie, H. Eur. J. Med. Chem. 2003, 38, 495.
[5] Prashantha, K. B. R.; Pankaj, M.; Karthikeyan, E.; Ankur, B.; Suja, P. V. Med. Chem. Res. 2010, 19, 344.
[6] Samzadeh, K. A.; Shafaroodi, H.; Miri, R.; Mirkhani, H. Med. Chem. Res. 2009, 18, 112.
[7] Kharkar, P. S.; Desai, B.; Gaveria, H. J. Med. Chem. 2002, 45, 4858.
[8] Cooper, K.; Fray, M. J.; Parry, M. J. J. Med. Chem. 1992, 35, 3115.
[9] Nasr, M.; Hoseini, S.; Montazerozohori, M.; Mehrabi, R.; Nasrabadi, H. J. Mol. Catal. A:Chem. 2014, 382, 99.
[10] Bitaraf, M.; Ali, A.; Otokesh, S. J. Chin. Chem. Soc. 2016, 63, 336.
[11] Tamaddon, F.; Ghazi, S. Catal. Commun. 2015, 72, 63.
[12] Guo, S.-R.; Yuan, Y.-Q.; Zhang, C.-N.; Wu, X.-M.; Sun, C. Chin. J. Org. Chem. 2010, 28, 811(in Chinese). (郭圣荣, 袁艳琴, 张春牛, 吴香梅, 孙晨, 有机化学, 2010, 28, 811.)
[13] Li, J.-P.; Chou, J.-K.; Li, H.-J.; Zhang, G.-S. Chin. J. Org. Chem. 2011, 29, 511(in Chinese). (李建平, 仇记宽, 李会娟, 张贵生, 有机化学, 2011, 29, 511.)
[14] Wang, D.-L.; Dong, Z.; Liu Z.; Zhao W.; Yang, F.-F. Chin. J. Org. Chem. 2014, 34, 783(in Chinese). (王道林, 董哲, 刘忠, 赵伟, 杨菲菲, 有机化学, 2014, 34, 783.)
[15] Pei, W.; Wang, Q.; Li, X.-N.; Sun, C. Chin. J. Chem. 2010, 28, 483.
[16] Nikoorazm, M.; Ghorbani, C. A.; Khanmoradi, M. RSC Adv. 2016, 6, 56549.
[17] Ananda, K. T.; Mohan, C. V. S.; Satyanarayana, K. Synth. Commun. 2014, 44, 574.
[18] Tabassum, S.; Govindaraju, S.; Khan, R.; Pasha, M. A. RSC Adv. 2016, 6, 29802.
[19] Khaskel, A.; Barman, P. Heteroat. Chem. 2016, 27, 114.
[20] Zhang, Z.; Zeng, X.; Xie, D.; Chen, D.; Ding, L,; Wang, A.; Yang, L.; Zhong, G. Org. Lett. 2015, 17, 5052.
[21] Jiang, Y.-H.; Yan, C.-G. Chin. J. Chem. 2016, 34, 1255.
[22] Lin, W.; Zheng, Y.-X.; Huang, Z.-B.; Shi, D.-Q. Chin. J. Org. Chem. 2017, 37, 508(in Chinese). (林伟, 郑永祥, 黄志斌, 史达清, 有机化学, 2017, 37, 508.)
[23] Shabalala, N.; Maddila, S.; Jonnalagadda, S. B. New J. Chem. 2016, 40, 5107.
[24] Kumar, A.; Sharma, S. Green Chem. 2011, 13, 2017.
[25] Wang, R.; Liu, X.; Wu, R.; Yu, B.; Li, H. RSC Adv. 2016, 6, 11.
[26] Xu, Q.-J.; Zhou, D.-P.; Cui, Y.-C. Chin. J. Org. Chem. 2007, 27, 1520(in Chinese). (徐启杰, 周大鹏, 崔元臣, 有机化学, 2007, 27, 1520.)
/
| 〈 |
|
〉 |