

Chinese Journal of Organic Chemistry >
Synthesis and Bioactivities of Multiheteocyclic Molecules Based on s-Triazine
Received date: 2017-01-12
Revised date: 2017-03-31
Online published: 2017-04-13
Supported by
Project supported by the Science and Technology Research Program of Liaoning Provincial Department of Education (No. 2009A426).
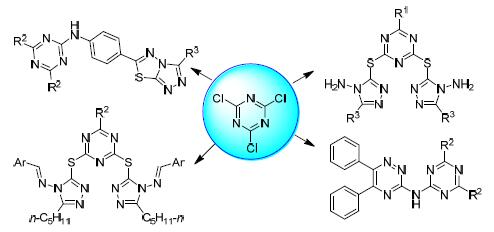
s-Triazine derivatives have good significant biological activities. It has been an important method to develop novel drugs by introduction of other heterocyclic rings onto s-triazine. Twenty-one novel target molecules were designed and synthesized by combination of 1,2,4-triazole, triazolo[3,4-b]thiadiazole and 1,2,4-triazine unit with s-triazine respectively. The structures of the target molecules were characterized by IR, 1H NMR and HRMS. In order to study the effect of different substituents on the efficacy activities, first, 8 target molecules containing double 1,2,4-triazole unit were synthesized by the condensation of four different mono-substituted s-triazines with intermediates containing n-pentyl and benzyl, respectively. Meanwhile, 4 target molecules were synthesized by the modification of the amino group. 9 target molecules were also afforded by the reaction of seven disubstituted s-triazines with the intermediates. The inhibitory activities of the 21 target molecules against Cdc25B were evaluated. The results showed that 13 target molecules exhibited good inhibitory activities against Cdc25B. The IC50 values were between (3.99±0.80)~(0.44±0.07) μg/mL, wherein the IC50 of 5 target molecules were lower than comparison reference Na3VO4, which were expected to be potential anticancer drugs.
Key words: s-triazine; 1,2,4-triazole; triazolo[3,4-b]thiadiazole; 1,2,4-triazine; Cdc25B
Liu Yaning , Sun Xiaona , Gao Ran , Li Chuanyin , Wang Jing , Li Yizheng , Zhang Chenglu . Synthesis and Bioactivities of Multiheteocyclic Molecules Based on s-Triazine[J]. Chinese Journal of Organic Chemistry, 2017 , 37(8) : 2057 -2065 . DOI: 10.6023/cjoc201701011
[1] Sharma, N.; Mohanakrishnan, D.; Shard, A.; Sharma, A.; Saima, A. K.; Sinha, D. Sahal J. Med. Chem. 2012, 55, 297.
[2] Saczewski, F.; Bu?akowska, A. Eur. J. Med. Chem. 2006, 41, 611.
[3] Zheng, M.; Xu, C.; Ma, J.; Sun, Y.; Du, F.; Liu, H.; Lin, L.; Li, C.; Ding, J.; Chen, K.; Jiang, H. Bioorg. Med. Chem. 2007, 15, 1815.
[4] Liu, K.; Xia, B.; Ma, W.; Zheng, B.; Zhang, X.; Fan, B. QSAR Comb. Sci. 2008, 27, 425.
[5] El-Faham, A.; Soliman, S. M.; Ghabbour, H. A.; Elnakady, Y. A.; Mohaya, T. A.; Siddiqui, M. R. H. J. Mol. Struct. 2016, 1125, 121.
[6] Bennett, G. B.; Mason, R. B.; Alden, L. J.; RoachJr, J. B. J. Med. Chem. 1978, 21, 623.
[7] Ojha, H.; Gahlot, P.; Tiwari, A. K.; Pathak, M.; Kakkar, R. Chem. Biol. Drug Des. 2011, 77, 57.
[8] Agarwal, A.; Srivastava, K.; Puri, S. K.; Chauhan, P. M. S. Bioorg. Med. Chem. Lett. 2005, 15(3), 531.
[9] Kapil, A.; Anshu, D. Bioorg. Med. Chem. 2007, 17, 3298.
[10] Mallon, R.; Feldberg, L. R.; Lucas, J.; Chaudhary, I.; Dehnhardt, C.; Santos, E. D.; Chen, Z.; Dos Santos, O.; Ayral-Kaloustian, S.; Venkatesan, A.; Hollander, I. Clin. Cancer Res. 2011, 17, 3193.
[11] Burger, M. T.; Pecchi, S.; Wagman, A.; Ni, Z. J.; Knapp, M.; Hendrickson, T.; Atallah, G.; Pfister, K.; Zhang, Y.; Bartulis, S.; Frazier, K.; Ng, S.; Smith, A.; Verhagen, J.; Haznedar, J.; Huh, K.; Iwanowicz, E.; Xin, X.; Menezes, D.; Merritt, H.; Lee, I.; Wiesmann, M.; Kaufman, S.; Crawford, K.; Chin, M.; Bussiere, D.; Shoemaker, K.; Zaror, I.; Maira, S. M.; Voliva, C. F. ACS. Med. Chem. Lett. 2011, 2, 774.
[12] Maira, S. M.; Pecchi, S.; Huang, A.; Burger, M.; Knapp, M.; Sterker, D.; Schnell, C.; Guthy, D.; Nagel, T.; Wiesmann, M.; Brachmann, S.; Fritsch, C.; Dorsch, M.; Chene, P.; Shoemaker, K.; Pover, A. D.; Menezes, D.; Martiny-Baron, G.; Fabbro, D.; Wilson, C. J.; Schlegel, R.; Hofmann, F.; Garc?a- Echeverr?a, C.; Sellers, W. R.; Voliva, C. F. Mol. Cancer. Ther. 2012, 11, 317.
[13] Sar?p?nar, E.; Gecen, N.; Sahin, K.; Yaamaz, E. Eur. J. Med. Chem. 2010, 45(9), 4157.
[14] Sunduru, N.; Gupta, L.; Chaturvedi, V.; Dwivedi, R.; Sinh, S.; Chauhan, P. M. S. Eur. J. Med. Chem. 2010, 45(8), 3335.
[15] Kong, D. X.; Yamori, I. Acta Pharmacol. Sin. 2010, 31, 1189.
[16] Badr, S. M. I.; Barwa, R. M. Bioorg. Med. Chem. 2011, 19, 4506.
[17] Goswami, B. N.; Kattaky, J. C. S.; Baruah, J. N. J. Heterocycl. Chem. 1984, 21, 1225.
[18] Seelam, N.; Shrivastava, S. P.; Prasanthi, S.; Gupta, S. J. Saudi Chem. Soc. 2016, 20, 411.
[19] Papakonstantinou-Garoufalias, S. S.; Tani, E.; Todoulou, O.; Papadaki-Valiraki, A.; Filippatos, E.; De Clercq, E.; Kourounakis, P. N. J. Pharm. Pharmacol. 1998, 50, 117.
[20] Turan-Zitouni, G.; Kaplancikli, Z. A.; Erol, K.; Kilic, F. S. Farmaco 1999, 54, 218.
[21] Zhang, Z. Y.; Sun, X. W.; Chu, C. H.; Zhao, L. J. Chin. Chem. Soc. 1997, 44, 535.
[22] Amir, M.; Harish, K.; Javed, S. A. Eur. J. Med. Chem. 2008, 43, 2056.
[23] Rzeski, W.; Matysiak, J.; Kandefer-Szerszen, M. Bioorg. Med. Chem. 2007, 15, 3201.
[24] Karabasanagouda, T.; Adhikari, A. V.; Shettey, N. S. Eur. J. Med. Chem. 2007, 42, 521.
[25] Padmavathi, V.; Reddy, G. S.; Padmaja, A.; Kondaiah, P.; Shazia, A. Eur. J. Med. Chem. 2009, 44, 2106.
[26] Shiradkar, M. R.; Padhalingappa, M. B.; Bhetalabhotala, S.; Akula, K. C.; Tupe, D. A.; Pinninti, R. R.; Thummanagoti, S. Bioorg. Med. Chem. 2007, 15, 6397.
[27] Webster, K. R.; Kimball, S. D.; Misra, R. N.; Xiao, H. Y.; Kim, K. S.; Lu, S. F.; Han, W. C.; Barbosa, S. A.; Hunt, J. T.; Rawlins, D. B.; Shan, W. F.; Ahmed, S. Z.; Qian, L. G.; Chen, B. C.; Zhao, R. L.; Bednarz, M. S.; Kellar, K. A.; Mulheron, J. G.; Batorsky, R.; Roongta, U.; Kamath, A.; Marathe, P.; Ranadive, S. A.; Sack, J. S.; Tokarski, J. S.; Pavletich, N. P.; Lee, F. Y. F. J. Med. Chem. 2004, 47, 1719.
[28] Misra, R. N.; Xiao, H.; Y.; Williams, D. K.; Kim, K. S.; Lu, S. F.; Keller, K. A.; Mulheron, J. G.; Batorsky, R.; Tokarski, J. S.; Sack, J. S.; Kimball, S. D.; Lee, F. Y.; Webster, K. R. Bioorg. Med. Chem. Lett. 2004, 14, 2973.
[29] Badr, S. M. I.; Barwa, R. M. Bioorg. Med. Chem., 2011, 19, 4506.
[30] Kumar, G. V. S.; Rajendraprasad, Y.; Mallikarjuna, B. P.; Chandrashekar, S. M.; Kistayya, C. Eur. J. Med. Chem. 2010, 45, 2063.
[31] Sui, Z. H.; Guan, J.; Hlasta, D. J.; Macielag, M. J.; Foleno, B. D.; Goldschmidt, R. M.; Loeloff, M. J.; Webb, G. C.; Barrett, J. F. Bioorg. Med. Chem. Lett. 1998, 8, 1929.
[32] Barton, B.; Gouwns, S.; Schaefer, M. C.; Zeelie, B. Org. Process Res. Dev. 2003, 7, 1071.
[33] Azenha, M. E. D.; Burrows, H. D.; Canle, M. L.; Coimbra, R.; Fernandez, M. I.; Garc?a, M. V.; Rodrigues, A. E.; Santaballa, J. A.; Steenken, S.; Santaballa, J. A. Chem. Commun. 2003, 112.
[34] Zhang, C. L.; Guo, Y.; Sun, L. J.; Wu, Y. F.; Zhu, C. A.; Qu, R. F.; Wang, X.; Chai, J. H. Chin. J. Appl. Chem. 2014, 31, 1419(in Chinese). (张成路, 国阳, 孙立杰, 武一菲, 朱长安, 曲瑞峰, 王雪, 柴金华, 应用化学, 2014, 31, 1419.)
[35] Zhang, C. L.; Wang, X; Hu, X.; Sun, L. J.; Qu, R. F.; Guo, Y.; Cai, J. H.; Zhu, C. A. Chem. J. Chin. Univ. 2015, 36, 463(in Chinese). (张成路, 王雪, 胡雪, 孙立杰, 曲瑞峰, 国阳, 柴金华, 朱长安, 高等学校化学学报, 2015, 36, 463.)
[36] Zhang, C. L.; Tang, J.; Yin, L. Y.; Xi, H.; Guo, Y.; Sun, L. J. Chin. J. Org. Chem. 2016, 36, 358(in Chinese). (张成路, 唐杰, 殷立莹, 袭焕, 国阳, 孙立杰, 有机化学, 2016, 36, 358.)
[37] Lavecchia, A.; Giovanni, C. D.; Pesapane, A.; Montuori, N.; Ragno, P.; Martucci, N. M.; Masullo, M.; Vendittis, E. D. J. Med. Chem. 2012, 55, 4142.
[38] Zhu, C. A. M.S. Thesis, Liaoning Normal University, Dalian, 2015(in Chinese). (朱长安, 硕士论文, 辽宁师范大学, 大连, 2015.)
[39] Chai, J. H. M.S. Thesis, Liaoning Normal University, Dalian, 2015(in Chinese). (柴金华, 硕士论文, 辽宁师范大学, 大连, 2015.)
[40] Sun, L. J. M.S. Thesis, Liaoning Normal University, Dalian, 2016(in Chinese). (孙立杰, 硕士论文, 辽宁师范大学, 大连, 2016.)
[41] Zhang, R. B.; Lu, J. R.; Xin, C. W.; Liu, J. B.; Mu, J. B.; Yang, X. Y.; Wang, H. Y.; Wang, M. J.; Zhang, H. Chin. J. Org. Chem. 2015, 35, 858(in Chinese). (张瑞波, 卢俊瑞, 辛春伟, 刘金彪, 穆江蓓, 杨旭云, 王宏韫, 王美君, 张贺, 有机化学, 2015, 35, 858.)
[42] Wang, B. L.; Shi, Y. X.; Zhan, Y. Z.; Zhang, L. Y.; Zhang, Y.; Wang, L. Z.; Zhang, X.; Li, Y. H.; Li, Z. M.; Li, B. J. Chin. J. Chem. 2015, 33, 1124.
/
| 〈 |
|
〉 |