

Chinese Journal of Organic Chemistry >
Synthesis and Properties of 4-Ferrocenyl-carboxybenzenecoumarin Derivatives
Received date: 2017-01-10
Revised date: 2017-04-07
Online published: 2017-04-21
Supported by
Project supported by the National Natural Science Foundation of China (No. 21171149).
Six novel ferrocenyl-carboxybenzene-coumarin derivatives were synthesized by 4-ferrocenylbenzoic and coumarin as raw materials through nitration, reduction reaction and condensation. The structures of compounds were characterized by IR, 1H NMR, 13C NMR and elemental analysis. The crystal structure of 4-ferrocene benzoic acid(coumarin-4-yl) ester (FcL1) was determined by X-ray diffraction analysis. The electrochemical research showed that the redox reaction on the surface of electrode was reversible with single electron and controlled by diffusion. Biological activity test results showed that the compound modified not conducive to the improvement of the bactericidal activity, only beneficial to the improvement of the antitumor activity. All the six compounds showed good inhibition against Curvularia lunata and Fusarium graminearum. In addition, FcL1 and 4-ferrocene benzoic acid (coumarin-7-yl) ester (FcL2) exhibited significant activities and selectivities against Esophageal carcinoma cell (IC50 value=2.10 and 1.25 μmol/L, respectively) in the anticancer activity test.
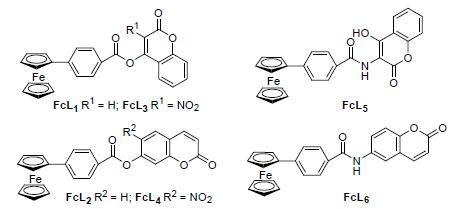
Key words: ferrocene; coumarin; electrochemical research; biological activity
Li Biao , Liu Qiuxi , Zhou Yuanqing , Jia Zhaodong , Zhu Manyu , Xu Yan , Song Maoping . Synthesis and Properties of 4-Ferrocenyl-carboxybenzenecoumarin Derivatives[J]. Chinese Journal of Organic Chemistry, 2017 , 37(8) : 2008 -2014 . DOI: 10.6023/cjoc201611036
[1] Wang, D.-L.; Yang, F.-F.; Liu, Z.; Dong, Z.; Zhao, W. Chin. J. Org. Chem. 2014, 34, 204(in Chinese). (王道林, 杨菲菲, 刘忠, 董哲, 赵伟, 有机化学, 2014, 34, 204.)
[2] Yang, S.-P.; Han, L.-J.; Pan, Y.; Wang, D.-Q.; Wang, N.-N.; Wang, T. Chem. J. Chin. Univ. 2013, 34, 364(in Chinese). (杨树平, 韩立军, 潘燕, 王大奇, 王南南, 王婷, 高等学校化学学报, 2013, 34, 364.)
[3] Shi, Y.; Zhou, C.-H.; Zhou, X.-D.; Geng, R.-X.; Ji, Q.-G. Acta. Pharm. Sin. 2011, 46, 798(in Chinese). (时园, 周成合, 周向东, 耿蓉霞, 吉庆刚, 药学学报, 2011, 46, 798.)
[4] Wang, D.; Wei, Y.; Hao, S.-H. Chin. J. Org. Chem. 2015, 35, 1691(in Chinese) (王栋, 魏艳, 郝双红, 有机化学, 2015, 35, 1691.)
[5] Liu, M.-M.; Chen, X.-Y.; Huang, Y.-Q.; Feng, P.; Guo, Y.-L.; Yang, G.; Chen, Y. J. Med. Chem. 2014, 57, 9343.
[6] Yeggoni, D. P.; Gokara, M.; Manidhar, D. M.; Rachamallu, A.; Nakka, S.; Reddy, C. S.; Subramanyam, R. Mol. Pharm. 2014, 11, 1117.
[7] Figueroa-Guiñez, R.; Matos, M. J.; Vazquez-Rodriguez, S.; Santana, L.; Uriarte, E.; Borges, F.; Olea-Azar, C.; Maya, J. D. Curr. Top. Med. Chem. 2015, 15, 850.
[8] Lei, Z.-L.; Hou, W.-C.; Luo, Y.-P. Chem. Reag. 2016, 38, 157(in Chinese). (雷震霖, 侯文成, 骆焱平, 化学试剂, 2016, 38, 157.)
[9] Wang, A.-L.; Tao, B.; Ai, C.-Z.; Zheng, X.-F. Chin. J. Org. Chem. 2015, 35, 843(in Chinese). (王爱玲, 陶波, 艾纯芝, 郑学仿, 有机化学, 2015, 35, 843.)
[10] Liu, M.; Liu, Y.; Liu, A.-L.; Zhang, D.-K.; Chen, M.-G.; Wu, C.-C.; Hua, X.-W.; Zhou, S.; Li, Z.-M. Chin. J. Org. Chem. 2016, 36, 1653(in Chinese). (刘明, 刘阳, 刘艾林, 张冬凯, 陈明桂, 吴长春, 华学文, 周莎, 李正名, 有机化学, 2016, 36, 1653.)
[11] Chen, H.; Zhou, L.-K.; Li, S.; Yao, Y.-C.; Gu, Y.-J.; Li, C.-X.; Li, N.; Meng, M.; Li, X.-L. Chin. J. Org. Chem. 2013, 33, 164(in Chinese). (陈华, 周利凯, 李帅, 姚玉超, 谷云景, 李春晓, 李娜, 孟明, 李小六, 有机化学, 2013, 33, 164.)
[12] Li, L.-H.; Chen, L.; Xia, Y.-F. J. China. Pharm. Univ. 2013, 44, 374(in Chinese). (李林虎, 陈莉, 夏玉凤, 中国药科大学学报, 2013, 44, 374.)
[13] Yang, S.-P.; Han, L.-J.; Pan, Y.; Wang, D.-Q.; Zhou, Y.-N.; Zhang, F. Sci. China, Ser. B 2013, 43, 858(in Chinese). (杨树平, 韩立军, 潘燕, 王大奇, 周亚男, 张凡, 中国科学B辑:化学, 2013, 43, 858.)
[14] Wang, D.-W.; Yu, X.; Yao, W.; Hu, W.-K.; Ge, C.-Y.; Shi, X.-D. Chem.-Eur. J. 2016, 22, 5543.
[15] Zhuang, H.; Zeng, R.-S.; Zou, J.-P. Chin. J. Chem. 2016, 34, 368.
[16] Wang, D.-W.; Ge, B.-Y.; Li, L.; Shan, J.; Ding, Y,-Q. J. Org. Chem. 2014, 79, 8607.
[17] Li, J.; Zeng, Y.; Zhang, X.-H; Yu, T.-J; Chen, J.-P.; Li, Y. Acta Chim. Sinica 2015, 73, 826(in Chinese). (李婧, 曾毅, 张小辉, 于天君, 陈金平, 李嫕, 化学学报, 2015, 73, 826.)
[18] Wang, D.-W.; Yu, X.-L.; Ge, B.-Y.; Miao, H.-Y.; Ding, Y.-Q. Chin. J. Org. Chem. 2015, 35, 676(in Chinese). (王大伟, 余晓丽, 葛冰洋, 苗红艳, 丁玉强, 有机化学, 2015, 35, 676.)
[19] Fan, W.; Li, M.; Hong, C.-Y.; Pan, C.-Y.; Acta Chim. Sinica 2015, 73, 330(in Chinese). (范溦, 李敏, 洪春雁, 潘才元, 化学学报, 2015, 73, 330.)
[20] Gacche, R. N.; Jadhav, S. G. J. Exp. Clin. Med. 2012, 4, 165.
[21] Yang, J.; Liu, G.-Y.; Dai, F.; Cao, X.-Y.; Kang, Y.-F.; Hu, L.-M.; Tang, J.-J.; Li, X.-Z.; Li, Y.; Jin, X.-L.; Zhou, B. Bioorg. Med. Chem. Lett. 2011, 21, 6420.
[22] Serra, S.; Chicca, A.; Delogu, G.; Vázquez-Rodríguez, S.; Santana, L.; Uriarte, E.; Casu, L.; Gertsch, J. Bioorg. Med. Chem. Lett. 2012, 22, 5791.
[23] Zhang, W.-H.; Jiang, M.-G. Chin. J. Org. Chem. 2010, 30, 254(in Chinese). (章维华, 蒋木庚, 有机化学, 2010, 30, 254).
[24] Vázquez, R.; Riveiro, M, E.; Vermeulen, M.; Alonso, E.; Mondillo, C.; Facorro, G.; Piehl, L.; Gómez, N.; Moglioni, A.; Fernández, N.; Baldi, A.; Shayo, C.; Davio, C. Bioorg. Med. Chem. 2012, 20, 5537.
[25] Kempen, I.; Papapostolou, D.; Thierry, N.; Pochet, L.; Counerotte, S.; Masereel, B.; Foidart, J. F.; Reboud-Ravaux, M.; Noel, A.; Pirotte, B. Brit. J. Cancer 2003, 88, 1111.
[26] Al-Amiery, A. A.; Al-Bayati, R. I. H.; Saour, K. Y.; Radi, M. F. Res. Chem. Intermed. 2012, 38, 559.
[27] Wu, K.-L.; Zhang, W.-B.; Zhou, D.; Xu, Y. Chin. J. Org. Chem. 2014, 34, 1201(in Chinese). (吴孔丽, 张吾斌, 周丹, 徐琰, 有机化学, 2014, 34, 1201.)
[28] Wang, D.-W.; Yu, X.-L.; Xu, X.; Ge, B.-Y.; Wang, X.-L.; Zhang, Y.-X. Chem.-Eur. J. 2016, 22, 8663.
[29] Pan, C.-X.; Wang, Z.-C.; Su, G.-F.; Zong, X.; Xue, W.-B.; Tan, J.-K. Chin. J. Org. Chem. 2012, 32, 742(in Chinese). (潘成学, 王忠长, 苏桂发, 宗玺, 薛文彬, 覃江克, 有机化学, 2012, 32, 742.)
[30] Liu, W.-H.; Wang, S.-B.; Chang, J.-X.; Liu, Y. Acta Pharm. Sin. 2014, 49, 217(in Chinese). (刘文虎, 王仕宝, 常晋霞, 刘毅, 药学学报, 2014, 49, 217.)
[31] (a) Tsutomu, I.; Hiroyuki, S.; Akira, O. JP 2004066547, 2004,[Chem. Abstr. 2004, 140, 225859].
(b) Rao, H. S.; Sivakumar, S. J. Org. Chem. 2006, 71, 8715.
(c) Verma, R. K.; Verma, G. K.; Shukla, G.; Singh, M. S. RSC Adv. 2012, 2, 2413.
(d) Wang, W.-F.; Fan, J.; Luo, C.-Z.; Yuan, Y.-F.; Zhang, Y.-F. ARKIVOC 2011, 44, 105.
[32] CCDC 1487759 contains the supplementary crystallographic data for coupound FcL1. These data can be obtained free of charge from The Cambridge Crystallographic Data Center via www.ccdc.cam.ac.uk/data_request/cif.
[33] Xu, Y.; Zhu, L.-M.; Ran, C.-L.; Wang, H.-X.; Fan, Y.-T. Chin. J. Inorg. Chem. 2007, 23, 589(in Chinese). (徐琰, 朱丽敏, 冉春玲, 王海先, 樊耀亭, 无机化学学报, 2007, 23, 589.)
[34] Chen, Y.-W; Wan, Y.-Y.; Liu, Q.-X.; Liu, J.-B.; Xiong, L.-X.; Yu, S.-J.; Li, Z.-M. Chin. J. Org. Chem. 2015, 35, 882(in Chinese). (陈有为, 万莹莹, 刘巧霞, 刘敬波, 熊丽霞, 于淑晶, 李正名, 有机化学, 2015, 35, 882.)
[35] Jiang, W.-T.; Hu, F.-Z.; Gu, H.; Liu, C.; Wei, N.-X.; Wan, L.; Ren, S.-Z.; Wang, J.-T.; Xu, F.-B. Chin. J. Org. Chem. 2014, 34, 774(in Chinese). (姜文涛, 胡方中, 顾翰, 刘传, 魏乃翔, 万蕾, 任士钊, 王俊婷, 徐凤波, 有机化学, 2014, 34, 774.)
[36] Zhang, H. X.; He, Q. Y. J. Pharm. Res. 2016, 35, 63(in Chinese). (张会鲜, 何琪杨, 药学研究, 2016, 35, 63.)
[37] Wang, C.; Jiang, R.-S.; Feng, F.; Fu, G.-L. Chem. Reag. 2008, 30, 935(in Chinese). (王诚, 江润生, 冯锋, 付国良, 化学试剂, 2008, 30, 935.)
[38] (a) Robertson, G. R.; Evans, R. A. J. Org. Chem. 1940, 5, 142.
(b) Li, M.-H.; Wang, J.; Fang, S.-D.; He, Z.-L. Chin. J. Spectrosc. Lab. 2001, 18, 63(in Chinese). (李明慧, 王井, 方世东, 何钟林, 光谱实验室, 2001, 18, 63.)
(c) Wang, D.-T; Wang, Y.-P. Mater. Sci. Technol. 2005, 13, 662(in Chinese). (王东田, 王郁萍, 材料科学与工艺, 2005, 13, 662.)
/
| 〈 |
|
〉 |