

Chinese Journal of Organic Chemistry >
Investigation on Preparation of p-Benzoquinone through the Organoselenium-Catalyzed Selective Oxidation of Phenol
Received date: 2017-01-12
Revised date: 2017-03-27
Online published: 2017-04-27
Supported by
Project supported by the National Natural Science Fundation of China (No. 21202141), the Natural Science Foundation of Zhejiang Province for Distinguished Young Scholars (No. LR14B020002), the Priority Academic Program Development of Jiangsu Higher Education Institutions and the High Level Talent Support Project of Yangzhou University.
Selective oxidation of phenol to produce p-benzoquinone is an important reaction with good industrial application value. The method for the synthesis of p-benzoquinone through the organoselenium-catalyzed selective oxidation of phenol with H2O2 is reported. Compared with known technologies, organoselenium-catalyzed oxidation reactions avoided the use of metal catalysts and the metal residue in products. The reaction procedures were very clean and were performed under mild conditions. By using this method, the selectivity of p-benzoquinone could reach 91.6% at the maximum.
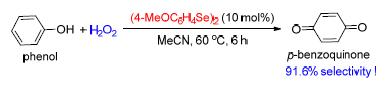
Key words: organoselenium catalysis; phenol; oxidation; p-benzoquinone; hydroquinone
Wang Fang , Xu Lin , Sun Cheng , Xu Qing , Huang Jiejun , Yu Lei . Investigation on Preparation of p-Benzoquinone through the Organoselenium-Catalyzed Selective Oxidation of Phenol[J]. Chinese Journal of Organic Chemistry, 2017 , 37(8) : 2115 -2118 . DOI: 10.6023/cjoc201701026
[1] Bai, W.-B.; Yu, X.-H.; Ding, W.; Xing, R.-T.; Liu, Y. Chem. Ent. Manag. 2013, 3, 84(in Chinese). (白卫兵, 余咸旱, 丁伟, 杏若婷, 刘源, 化工管理, 2013, 3, 84.)
[2] (a) Kanie, S.; Nishikawa, T.; Ojika, M.; Oba, Y. Sci. Rep. 2016, 6, 24794.
(b) Papa, C. M.; Cesnik, A. J.; Evans, T. C.; Choi, K.-S. Langmuir 2015, 31, 9502.
(c) Kunitsa, A. A.; Bravaya, K. B. J. Phys. Chem. Lett. 2015, 6, 1053.
(d) Stejskal, J.; Bober, P.; Trchová, M.; Horský, J.; Pilar, J.; Walterová, Z. Synth. Met. 2014, 192, 66.
[3] (a) Gharah, N.; Chakraborty, S.; Mukherjee, A. K.; Bhattacharyya, R. Inorg. Chim. Acta 2009, 362, 1089.
(b) Maiti, S. K.; Banerjee, S.; Mukherjee, A. K.; Abdul Malik, K. M.; Bhattacharyya, R. New J. Chem. 2005, 29, 554.
(c) Maiti, S. K.; Abdul Malik, K. M.; Bhattacharyya, R. Inorg. Chem. Commun. 2004, 7, 823.
(d) Wong, W.-K.; Chen, X.-P.; Chik, T.-W.; Wong, W.-Y.; Guo, J.-P.; Lee, F.-W. Eur. J. Inorg. Chem. 2003, 3539.
[4] (a) Cheng, W.-J.; Jiang, Y.-Q.; Xu, X.-Z.; Wang, Y.; Lin, K.-F.; Pescarmona, P. P. J. Catal. 2016, 333, 139.
(b) Inagaki, S.; Tsuboi, Y.; Sasaki, M.; Mamiya, K.; Park, S.; Kubota, Y. Green Chem. 2016, 18, 735.
(c) Wang, B.-R.; Lin, M.; Zhu, B.; Peng, X.-X.; Xu, G.-T.; Shu, X.-T. Catal. Commun. 2016, 75, 69.
(d) Maneesuwan, H.; Tantisriyanurak, S.; Chaisuwan, T.; Wongkasemjit, S. Appl. Catal. A 2015, 504, 448.
[5] (a) Santoro, S.; Azeredo, J. B.; Nascimento, V.; Sancineto, L.; Braga, A. L.; Santi, C. RSC Adv. 2014, 4, 31521.
(b) Freudendahl, D. M.; Santoro, S.; Shahzad, S. A.; Santi, C.; Wirth, T. Angew. Chem., Int. Ed. 2009, 48, 8409.
[6] (a) Guo, R.-Z.; Huang, J.-C.; Huang, H.-Y.; Zhao, X.-D. Org. Lett. 2016, 18, 504.
(b) Zhang, X.-L.; Guo, R.-Z.; Zhao, X.-D. Org. Chem. Front. 2015, 2, 1334.
(c) Cresswell, A. J.; Eey, S. T.-C.; Denmark, S. E. Catal. Nat. Chem. 2015, 7, 146.
(d) Chen, F.; Tan, C. K.; Yeung, Y.-Y. J. Am. Chem. Soc. 2013, 135, 1232.
[7] (a) Jing, X.-B.; Yuan, D.-D.; Yu, L. Adv. Synth. Catal. 2017, 357, 1194.
(b) Wang, Y.-G.; Yu, L.-H.; Zhu, B.-C.; Yu, L. J. Mater. Chem. A 2016, 4, 10828.
(c) Yu, L.; Chen, F.-L.; Ding, Y.-H. ChemCatChem 2016, 8, 1033.
(d) Yu, L.; Bai, Z.-B.; Zhang, X.; Zhang, X.-H.; Ding, Y.-H.; Xu, Q. Catal. Sci. Technol. 2016, 6, 1804.
(e) Zhang, X.; Sun, J.-J.; Ding, Y.-H.; Yu, L. Org. Lett. 2015, 17, 5840.
(f) Zhang, X.; Ye, J.-Q.; Yu, L.; Shi, X.-K.; Zhang, M.; Xu, Q.; Lautens, M. Adv. Synth. Catal. 2015, 357, 955.
(g) Yu, L.; Ye, J.-Q.; Zhang, X.; Ding, Y.-H.; Xu, Q. Catal. Sci. Technol. 2015, 5, 4830.
(h) Yu, L.; Li, H.-Y.; Zhang, X.; Ye, J.-Q.; Liu, J.-P.; Xu, Q.; Lautens, M. Org. Lett. 2014, 16, 1346.
(i) Yu, L.; Wu, Y.-L.; Cao, H.-E.; Zhang, X.; Shi, X.-K.; Luan, J.; Chen, T.; Pan, Y.; Xu, Q. Green Chem. 2014, 16, 287.
(j) Yu, L.; Wang, J.; Chen, T.; Wang, Y.-G.; Xu, Q. Appl. Organomet. Chem. 2014, 28, 652.
(k) Yu, L.; Wang, J.; Chen, T.; Ding, K-H.; Pan, Y. Chin. J. Org. Chem. 2013, 33, 1096(in Chinese) (俞磊, 王俊, 陈天, 丁克鸿, 潘毅, 有机化学, 2013, 33, 1096.)
[8] (a) Santoro, S.; Santi, C.; Sabatini, M.; Testaferri, L.; Tiecco, M. Adv. Synth. Catal. 2008, 350, 2881.
(b) Sancineto, L.; Tidei, C.; Bagnoli, L.; Marini, F.; Lenardao, E. J.; Santi, C. Molecules 2015, 20, 10496.
[9] (a) Deng, S.-C.; Meng, T.-T.; Xu, B.-L.; Gao, F.; Ding, Y.-H.; Yu, L.; Fan, Y.-N. ACS Catal. 2016, 6, 5807.
(b) Yu, L.; Han, Z.; Ding, Y.-H. Org. Process Res. Dev. 2016, 20, 2124.
(c) Deng, S.-C.; Zhuang, K.; Xu, B.-L.; Ding, Y.-H.; Yu, L.; Fan, Y.-N. Catal. Sci. Technol. 2016, 6, 1772.
(d) Yu, L.; Han, M.-T.; Luan, J.; Xu, L.; Ding, Y.-H.; Xu, Q. Sci. Rep. 2016, 6, 30432.
(e) Xu, L.; Wang, F.; Huang, J.-J.; Xu, Q.; Yu, L.; Fan, Y.-N. Chin. J. Org. Chem. 2016, 36, 2232(in Chinese). (徐林, 王芳, 黄杰军, 徐清, 俞磊, 范以宁, 有机化学, 2016, 36, 2232.)
(f) Yu, L.; Huang, Y.-P.; Wei, Z.; Ding, Y.-H.; Su, C.-L.; Xu, Q. J. Org. Chem. 2015, 80, 8677.
(g) Yu, L.; Wu, Y.-L.; Chen, T.; Pan, Y.; Xu, Q. Org. Lett. 2013, 15, 144.
(h) Yu, L.; Wang, J.; Cao, H.-E.; Ding, K.-H.; Xu, Q. Chin. J. Org. Chem. 2014, 34, 1986(in Chinese). (俞磊, 王俊, 曹洪恩, 丁克鸿, 徐清, 有机化学, 2014, 34, 1986.)
[10] Jin, W.-W.; Zheng, P.; Wong, W.-T.; Law, G.-L. Adv. Synth. Catal. 2017, 359, 1588.
[11] In our previous investigations on the organoselenium-catalyzed oxidation of cyclohexene, small amount of cyclohex-2-en-1-ol and cyclohex-2-en-1-one were also detected as the by-products, indicating the existence of the dehydration step.
[12] van der Toorn, J. C.; Kemperman, G.; Sheldon, R. A.; Arends, I. W. C. E. J. Org. Chem. 2009, 74, 3085.
[13] (a) Niyomura, O.; Cox, M.; Wirth, T. Synlett 2006, 251.
(b) Browne, D. M.; Niyomura, O.; Wirth, T. Org. Lett. 2007, 9, 3169.
(c) Singh, F. V.; Wirth, T. Org. Lett. 2011, 13, 6504.
[14] Patel, S.; Kuanar, M.; Nayak, B. B.; Banichul, H.; Mishra, B. K. Synth. Commun. 2005, 35, 1033.
/
| 〈 |
|
〉 |