

Chinese Journal of Organic Chemistry >
Progress on Synthesis and Application of Triazole-Based Tetrathiafulvalene Derivatives
Received date: 2017-02-03
Revised date: 2017-04-07
Online published: 2017-04-27
Supported by
Project supported by the National Natural Science Foundation of China (No. 21172105).
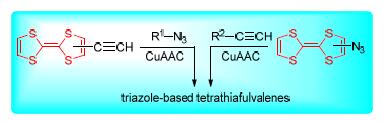
Tetrathiafulvalene (TTF) is an excellent electron donor, therefore it has been used in amyriad of molecular conductors and supramolecular application. The main focus of this review is on the recent progress of synthesis and application of various triazole-based TTF derivatives (TTFs) mainly including traditional tetrathiafulvalene, tetrathiafulvalene vinylogue and extended tetrathiafulvalene via click chemistry. Assisting copper(I)-catalyzed azide-alkyne cycloaddition reaction (CuAAC), the general aspects of TTF molecular design are mainly involved in the reactions of terminal propargyl TTFs with terminal azided substrates as well as the reactions of terminal azided TTFs with terminal propargyl various substrates. This survey is also presented from the view of supramolecular application of the triazole-based TTF systems in molecular recognition, molecular assembly as well as molecular photoelectric and photovoltaic functional materials etc.
Chen Xiaoji , Zhao Bangtun , Zhu Weimin , Tao Jingjing , Chen Xiaoji , Zhu Weimin . Progress on Synthesis and Application of Triazole-Based Tetrathiafulvalene Derivatives[J]. Chinese Journal of Organic Chemistry, 2017 , 37(8) : 1964 -1977 . DOI: 10.6023/cjoc201702002
[1] Segura, J. L.; Martin, N. Angew. Chem., Int. Ed. 2001, 40, 1372.
[2] Canevet, D.; Sallé, M.; Zhang, G. X.; Zhang, D. Q.; Zhu, D. B. Chem. Commun. 2009, 45, 2245.
[3] Pop, F.; Avarvari, N. Chem. Commun. 2016, 52, 7906.
[4] Bendikov, M.; Wudl, F.; Perepichka, D. F. Chem. Rev. 2004, 104, 4891.
[5] Zhu, Y. L.; Cao, L.; Ma, K. R.; Tian, L. B.; Wang, X. L.; Su, Z. M. Chem. J. Chin. Univ. 2013, 34, 952.
[6] Bouzan, S.; Chen, G.; Mulla, K.; Dawe, L.N.; Zhao, Y. M. Org. Biomol. Chem. 2012, 10, 7673.
[7] Brunetti, F. G.; López, J. L.; Atienza, C.; Martín, N. J. Mater. Chem. 2012, 22, 4188.
[8] Yamada, J.; Sugimoto, T. TTF Chemistry Fundamentals and Applications of Tetrathiafulvalene, Kodansha and Springer, Tokyo, 2004.
[9] Feng, M.; Gao, L.; Deng, Z. T.; Ji, W.; Guo, X. F.; Du, S. X.; Shi, D. X.; Zhang, D. Q.; Zhu, D. B.; Gao, H. J. J. Am. Chem. Soc. 2007, 129, 2204.
[10] Chen, T.; Liu, W. J.; Cong, Z. Q.; Yin, B. Z. Chin. J. Org. Chem. 2005, 25, 570(in Chinese). (陈铁, 刘武军, 丛志奇, 尹炳柱, 有机化学, 2005, 25, 570.)
[11] Zhu, Y. L.; Yang, Y. J.; Yin, Q. F.; Zhu, D. B. Chin. J. Org. Chem. 2005, 25, 1167(in Chinese). (朱玉兰, 杨艳杰, 尹起范, 朱道本, 有机化学, 2005, 25, 1167.)
[12] Huo, J. P.; Wei, X. P.; Mo, G. Z.; Peng, P.; Zhong, M. L.; Chen, R. H.; Wang, Z. Y. Chin. J. Org. Chem. 2014, 34, 92(in Chinese). (霍景沛, 韦新平, 莫广珍, 彭湃, 钟铭丽, 陈任宏, 汪朝阳, 有机化学, 2014, 34, 92.)
[13] Rostovtsev, V. V.; Green, L. G.; Fokin, V. V.; Sharpless, K. B. Angew. Chem., Int. Ed. 2002, 41, 2596.
[14] Tornøe, C. W.; Christensen, C.; Meldal, M. J. Org. Chem. 2002, 67, 3057.
[15] Kolb, H. C.; Finn, M. G.; Sharpless, K. B. Angew. Chem., Int. Ed. 2001, 40, 2004.
[16] Moses, J. E.; Moorhouse, A. D. Chem. Soc. Rev. 2007, 36, 1249.
[17] Wu, P.; Fokin, V. V. Aldrichim. Acta 2007, 40, 7.
[18] Dondoni, A. Chem. Asian J. 2007, 2, 700.
[19] Meldal, M.; Tornøe, C. W. Chem. Rev. 2008, 108, 2952.
[20] Huisgen, R.; Szeimies, G.; Mobius, L. Chem. Ber. 1967, 100, 2494.
[21] Lutz, J. F. Angew. Chem., Int. Ed. 2007, 46, 1018.
[22] Angell, Y. L.; Burgess, K. Chem. Soc. Rev. 2007, 36, 1674.
[23] Sumerlin, B. S.; Vogt, A. P. Macromolecules 2010, 43, 1.
[24] Hahn, U.; Elhabiri, M.; Trabolsi, A.; Herschbach, H.; Leize,E.; Dorsselaer, A. V.; Albrecht-Gary, A. M.; Nierengarten, J. F. Angew. Chem., Int. Ed. 2005, 44, 5338.
[25] Fournier, D.; Hoogenboom, R.; Schubert, U. S. Chem. Soc. Rev. 2007, 36, 1369.
[26] Biet, T.; Cauchy, T.; Avarvari, N. Chem. Eur. J. 2012, 18, 16097.
[27] Biet, T.; Avarvari, N. Org. Biomol. Chem. 2014, 12, 3167.
[28] Biet, T.; Avarvari, N. CrystEngComm 2014, 16, 6612.
[29] Salinas, Y.; Solano, M. V.; Sørensen, R. E.; Larsen, K. R.; Lycoops, J.; Jeppesen, J. O.; Martinez-Manez, R.; Sancenon, F.; Marcos, M. D.; Amorós, P.; Guillem. C. Chem.-Eur. J. 2014, 20, 855.
[30] Zhou, Y. C.; Zhang, D. Q.; Zhu, L. Y.; Shuai, Z. G.; Zhu, D. B. J. Org. Chem. 2006, 71, 2123.
[31] Li, X. H.; Zhang, G. X.; Ma, H. M.; Zhang, D. Q.; Li, J.; Zhu, D. B. J. Am. Chem. Soc. 2004, 126, 11543.
[32] Mulla, K.; Dongare, P.; Thompson, D. W.; Zhao, Y. M. Org. Biomol. Chem. 2012, 10, 2542.
[33] Mulla, K.; Dongare, P.; Thompson, D. W.; Zhao, Y. M. Org. Lett. 2013, 17, 4532.
[34] Jelinek, R.; Kolusheva, S. Chem. Rev. 2004, 104, 5987.
[35] Edwards, N. Y.; Sager, T. W.; McDevitt, J. T.; Anslyn, E. V. J. Am. Chem. Soc. 2007, 129, 13575.
[36] Tan, W.; Zhang, D.; Wang, Z.; Liu, C.; Zhu, D. J. Mater. Chem. 2007, 17, 1964.
[37] Shoji, E.; Freund, M. S. J. Am. Chem. Soc. 2002, 124, 12486.
[38] Mulla, K.; Zhao, Y. M. Tetrahedron Lett. 2014, 55, 382.
[39] Shao, M.; Zhao, Y. M. Tetrahedron Lett. 2010, 51, 2508.
[40] Qvortrup, K.; Petersen, M. A.; Hassenkam, T.; Nielsen, M. B. Tetrahedron Lett. 2009, 50, 5613.
[41] Zhao, B. T.; Liu, L. W.; Li, X. C.; Qu, G. R. Chin. J. Chem. 2012, 30, 254.
[42] Zhao, B. T.; Liu, L. W.; Li, X. C.; Qu, G. R.; Belhadj, E.; Le Derf, F.; Sallé, M. Tetrahedron Lett. 2013, 54, 23.
[43] Zhao, B. T.; Cao, S. N.; Guo, H. M.; Qu, G. R. Synth. Met. 2013, 174, 14.
[44] Liu, L. W.; Guo, W. B.; Li, X. C.; Qv, G. R.; Zhao, B. T. Chin. J. Org. Chem. 2010, 30, 1960(in Chinese). (刘连委, 郭文博, 李晓川, 渠桂荣, 赵邦屯, 有机化学, 2010, 30, 1960.)
[45] Zhao, B. T.; Zhu, X. M.; Chen, X. H.; Yan, Z. N. Chin. Chem. Lett. 2013, 24, 573.
[46] Zhao, B. T.; Peng, Q. M.; Zhu, X. M.; Yan, Z. N.; Zhu, W. M. J. Org. Chem. 2015, 80, 1052.
[47] Xue, M.; Yang, Y.; Chi, X. D.; Yan, X. Z.; Huang, F. H. Chem. Rev. 2015, 115, 7398.
[48] Spruell, J. M.; Paxton,W. F.; Olsen, J. C.; Benítez, D.; Tkatchouk, E.; Stern, C. L.; Trabolsi,A.; Friedman, D. C.; Goddard, W. A.; Stoddart, J. F. J. Am. Chem. Soc. 2009, 131, 11571.
[49] Fahrenbach, A. C.; Hartlieb, K. J.; Sue, C. H.; Bruns, C. J.; Barin, G.; Basu, S.; Olson, M. A.; Botros, Y. Y.; Bagabas, A.; Khdaryad, N. H.; Stoddart, J. F. Chem. Commun. 2012, 48, 9141.
[50] Andersen,S. S.; Share, A. I.; Poulsen, B. C.; Kørner, M.; Duedal, T.; Benson, C. R.; Hansen, S. W.; Jeppesen, J. O.; Flood, A. H. J. Am. Chem. Soc. 2014, 136, 6373.
[51] Baggerman, J.; Haraszkiewicz, N.; Wiering, P. G.; Fioravanti, G.; Marcaccio, M.; Paolucci, F.; Kay, E. R.; Leigh, D. A.; Brouwer, A. M. Chem.-Eur. J. 2013, 19, 5566.
[52] Avellini, T.; Li, H.; Coskun, A.; Barin, G.; Trabolsi, A.; Basuray, A. N.; Dey, S. K.; Credi, A.; Silvi, S.; Stoddart, J. F.; Venturi, M. Angew. Chem., Int. Ed. 2012, 51, 1611.
[53] Aprahamian, I.; Dichtel, W. R.; Ikeda, T.; Heath, J. R.; Stoddart, J. F. Org. Lett. 2007, 7, 1287.
[54] Aprahamian, I.; Yasuda, T.; Ikeda, T.; Saha, S.; Dichtel, W. R.; Isoda, K.; Kato, T.; Stoddart, J. F. Angew. Chem., Int. Ed. 2007, 46, 4675.
[55] Aprahamian, I.; Olsen, J. C.; Trabolsi, A.; Stoddart, J. F. Chem.-Eur. J. 2008, 14, 3889.
[56] Zhao, Y. L.; Dichtel, W. R.; Trabolsi, A.; Saha, S.; Aprahamian, I.;Stoddart, J. F. J. Am. Chem. Soc. 2008, 130, 11294.
[57] Zhang, Q.; Tu, Y, Q.; Tian, H.; Zhao, Y. L.; Stoddart, J. F.; Ågren, H. J. Phys. Chem. B 2010, 114, 6561.
[58] Barin, G.; Coskun, A.; Friedman, D. C.; Mark, A.; Olson, D.; Michael, T.; Colvin, R.; Sanjeev, K.; Dey, M. A.; Altan, B.; Michael, R.; Wasielewski, G.; Stoddart, J. F. Chem.-Eur. J. 2011, 17, 213.
[59] Kaminska, I.; Barras, A.; Coffinier, Y.; Lisowski, W.; Roy, S.; Niedziolka-Jonsson, J.; Woisel, P.; Lyskawa, J.; Opallo, M.; Siriwardena, A.; Boukherroub, R.; Szunerits, S. ACS Appl. Mater. Interf. 2012, 4, 5386.
[60] Selhorst, R. C.; Puodziukynaite, E.; Dewey, J. A.; Wang,P. J.; Barnes, M. D.; Ramasubramaniam, A.; Emrick, T. Chem. Sci. 2016, 7, 4698.
[61] Mateos-Gil, J.; Rodríguez-Pérez, L.; Guldi, D. M.; Herranz, M. A.; Martín, N. Nanoscale 2015, 7, 1193.
/
| 〈 |
|
〉 |