

Chinese Journal of Organic Chemistry >
Development on Application of Phenazine Derivatives in Molecular Recognition and Self-assembly
Received date: 2017-03-17
Revised date: 2017-05-13
Online published: 2017-06-02
Supported by
Project supported by the National Natural Science Foundation of China (Nos.21662031,21661018,21574104,21262032).
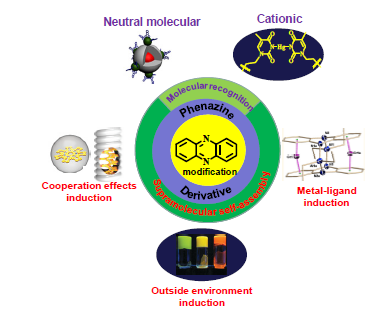
A wide variety of phenazine compounds are no stranger to organic chemistry researchers, it widely exists in organic natural products together with good biological activity. The synthetic process is simple and the functionalization of molecular structure is comparatively easy of phenazine compounds with natural skeleton. These compounds with multiple sites and large conjugated system, which make it easy to form hydrogen bond, ionic bond and π-π interaction and so on. Therefore, the phenazine compounds have extensive application in supramolecular chemistry. Molecular recognition (MR) and supramolecular self-assembly (MS-A) are two important research direction of supramolecular chemistry. The advances in the research of the development on application of phenazine derivatives in MR and MS-A in recent years are highlighted. According to different type of guest, the MR is grouped into three categories, including anion recognition (AR), cationic recognition (CR) and neutral molecular recognition (NMR). According to the difference of induction factors between guest and phenazine derivatives, the MS-A is grouped into four categories, including self-assembly induced by hydrogen bonding (HBSA), self-assembly induced by accumulation (ASA), self-assembly induced by metal-ligand (M-LSA), self-assembly induced by cooperation of multiple factors (MFSA), and self-assembly induced by the outside environment (OESA).
Li Wenting , Qu Wenjuan , Zhang Haili , Li Xiang , Lin Qi , Yao Hong , Zhang Youming , Wei Taibao . Development on Application of Phenazine Derivatives in Molecular Recognition and Self-assembly[J]. Chinese Journal of Organic Chemistry, 2017 , 37(10) : 2619 -2639 . DOI: 10.6023/cjoc201703023
[1] Chowdhury, G.; Sarkar, U.; Pullen, S.; Wilson, W. R.; Rajapakse, A.; Fuchsknotts, T.; Fuchs-Knotts, T.; Gates, S. Chem. Res. Toxicol. 2012, 25, 197.
[2] Saleh, O.; Bonitz, T.; Flinspach, K.; Kulik, A.; Burkard, N.; Mühlenweg, A.; Andreas, V.; Stefan, P.; Michael. L.; Bertolt, G.; Hans-Peter, F.; Lutz, H. Med. Chem. Commun. 2012, 3, 1009.
[3] Zhi, X.; Yang, C.; Zhang, R.; Hu, Y.; Ke, Y.; Xu, H. Ind. Crops Prod. 2013, 42, 520.
[4] Marler, L.; Condasheridan, M.; Cinelli, M. A.; Morrell, A. E.; Cushman, M.; Chen, L.; Huang. K.; Van, B. R.; Pezzuto, J. M. Anticancer Res. 2010, 30, 4873.
[5] Gerardo, P.; Marco, M.; Aida, R.; Anna, A.; Astolfo, Z.; Alessio, C.; Antonio, E. Nat. Prod. Res. 2013, 27, 956.
[6] Conda-Sheridan, M.; Udumula, V.; Endres, J. L.; Harper, C. N.; Jaramillo, L.; Zhong, H. A.; Kenneth, W. B.; Martin, C.-S. Eur. J. Med. Chem. 2016, 125, 710.
[7] Koot, D.; Cromarty, D. Drug Delivery Transl. Res. 2015, 5, 257.
[8] Cimmino, A.; Evidente, A.; Mathieu, V.; Andolfi, A.; Lefranc, F.; Kornienko, A.; Kiss, R. Nat. Prod. Res. 2012, 29, 487.
[9] Cloonan, S. M.; Elmes, R. B. P.; Erby, M. L.; Bright, S. A.; Poynton, F. E.; Nolan, D. E.; Quinn, S. J.; Gunnlaugsson, T.; Williams, D. C. J. Med. Chem. 2015, 58, 4494.
[10] Haas, D.; Blumer, C.; Keel, C. Curr. Opin. Biotechnol. 2000, 11, 290.
[11] Laursen, J.; Nielsen, J. Chem. Rev. 2004, 104, 1663.
[12] Bunz, U. H. F. Chem. Eur. J. 2009, 15, 6780.
[13] Bunz, U. H. F.; Engelhart, J. U.; Lindner, B. D.; Schaffroth, M. Angew. Chem., Int. Ed. 2013, 52, 3810.
[14] Miao, Q. Adv. Mater. 2014, 26, 5541.
[15] Xue, H.; Tang, X. J.; Wu, L. Z.; Zhang, L. P.; Tung, C. H. J. Org. Chem. 2005, 70, 9727.
[16] Brombosz, S. M.; Zucchero, A. J.; Phillips, R. L.; Vazquez, D.; Wilson, A.; Bunz, U. H. F. Org. Lett. 2007, 9, 4519.
[17] Feng, X. J.; Tian, P. Z.; Xu, Z.; Chen, S. F.; Wong, M. S. J. Org. Chem. 2013, 78, 11318.
[18] Gill, M. R.; Cecchin, D.; Walker, M. G.; Mulla, R. S.; Battaglia, G.; Smythe, C.; Thomas, J. A. Chem. Sci. 2013, 4, 4512.
[19] Yang, L.; Li, X.; Yang, J.; Qu, Y.; Hua, J. ACS Appl. Mater. Interfaces 2013, 5, 1317.
[20] Edwardson, T. G.; Lau, K. L.; Bousmail, D.; Serpell, C. J.; Sleiman, H. F. Nat. Chem. 2016, 8, 162.
[21] Bisker, G.; Dong, J.; Park, H. D.; Iverson, N. M.; Ahn, J.; Nelson, J. T.; Landry, M. P.; Kruss, S.; Strano, M. S. Nat. Commun. 2016, 7, 10241.
[22] Rónavári, A.; Kovács, D.; Vágvölgyi, C.; Kónya, Z.; Kiricsi, M.; Pfeiffer, I. J. Basic Microbiol. 2016, 56, 557.
[23] Shi, B. B.; Zhang, Y. M.; Wei, T. B.; Lin, Q.; Yao, H.; Zhang, P.; You, X. M. Sens. Actuators, B:Chem. 2014, 190, 555.
[24] Gao, G. Y.; Qu, W. J.; Shi, B. B.; Zhang, P.; Lin, Q.; Yao, H.; Yang, W. L.; Zhang, Y. M.; Wei, T. B. Sens. Actuators, B:Chem. 2014, 26, 39.
[25] Li, W. T.; Wu, G. Y.; Qu, W. J.; Li, Q.; Lou, J. C.; Qu, W. J.; Yao, H.; Zhang, Y. M.; Wei, T. B. Sens. Actuators, B:Chem. 2017, 239, 671.
[26] Wei, T. B.; Wu, G. Y.; Shi, B. B.; Lin, Q.; Yao, H.; Zhang, Y. M. Chin. J. Chem. 2014, 32, 1238.
[27] Zhang, P.; Zhang, Y.; Lin, Q.; Yao, H.; Wei, T. Chin. J. Org. Chem. 2014, 34, 1300(in Chinese). (张鹏, 张有明, 林奇, 姚虹, 魏太保, 有机化学, 2014, 34, 1300.)
[28] Bryant, J. J.; Zhang, Y.; Lindner, B. D.; Davey, E. A.; Appleton, L. A.; Qian, X.; Bunz, U. H. F. J. Org. Chem. 2012, 77, 7479.
[29] Li, G.; Gao, J. K.; Zhang, Q. C. Asian J. Org. Chem. 2014, 3, 203.
[30] Jardim, G. A. M.; Calado, H. D. R.; Cury, L. A.; Júnior, E. N. S. Eur. J. Org. Chem. 2015, 4, 703.
[31] Qi, G.; Fu, C.; Chen, G.; Xu, S; Xu, W. RSC Adv. 2015, 5, 49759.
[32] Zhou, H.; Sun, L.; Chen, W.; Tian, G.; Chen, Y.; Li, Y.; Su, J. Tetrahedron 2016, 72, 2300.
[33] Zhou, H.; Mei, J.; Chen, Y. A.; Chen, C. L.; Chen, W.; Zhang, Z.; Su, J.; Chou, P. T.; Tian, H. Small 2016, 12, 6542.
[34] Gao, G. Y.; Qu, W. J.; Shi, B. B.; Lin, Q.; Yao, H.; Zhang, Y. M.; Chang, J.; Cai, Y.; Wei, T. B. Sens. Actuators, B:Chem. 2015, 213, 501.
[35] Li, W.-T.; Wu, G.-Y.; Qu, W.-J.; Li, Q.; Lou, J.-C.; Lin, Q.; Yao, H.; Zhang, Y.-M.; Wei, T.-B. Sens. Actuators, B:Chem. 2017, 239, 671.
[36] Shive, M. S. C.; Tanuja, B.; Bhaskar, G. Tetrahedron Lett. 2008, 49, 6646.
[37] Wang, C.; Li, G.; Zhang, Q. Tetrahedron Lett. 2013, 54, 2633.
[38] Yang, L.; Li, X.; Yang, J.; Qu, Y.; Hua, J. ACS Appl. Mater. Interfaces 2013, 5, 1317.
[39] Yang, L.; Li, X.; Qu, Y.; Qu, W.; Zhang, X.; Hang, Y.; Ågren, H.; Hua, J. Sens. Actuators, B:Chem. 2014, 203, 833.
[40] Li, G.; Wu, Y.; Gao, J.; Li, J.; Zhao, Y.; Zhang, Q. Chem. Asian J. 2013, 8, 1574.
[41] Xu, Q.; Heo, C. H.; Kim, G.; Lee, H. W.; Kim, H. M.; Yoon, J. Angew. Chem., Int. Ed. 2015, 54, 4890.
[42] Wei, T.-B.; Li, W.-T.; Li, Q.; Su, J.-X.; Qu, W.-J.; Lin, Q.; Yao, H.; Zhang, Y-M. Tetrahedron Lett. 2016, 57, 2767
[43] Li, X.; Lin, Q.; Qu, W.; Li Q.; Chen, X.; Li, W.; Zhang, Y.; Yao, H.; Wei, T. Chin. J. Org. Chem. 2017, 37, 889(in Chinese). (李翔, 林奇, 曲文娟, 李乔, 程晓斌, 李文婷, 张有明, 姚虹, 魏太保, 有机化学, 2017, 37, 889.)
[44] Wei, T. B.; Li, W. T.; Li, Q.; Qu, W. J.; Li, H.; Yan, G. T.; Li, Q.; Yao, H.; Zhang, Y. M. RSC Adv. 2016, 6, 43832.
[45] Li, W.-T.; Qu, W.-J.; Zhu, X.; Li, Q.; Zhang, H.-L.; Yao, H.; Lin, Q.; Zhang, Y.-M.; Wei, T.-B. Sci. China, Chem. 2017, 60, 754.
[46] Zhang, H. L; Wei, T. B.; Li, W. T.; Qu, W. J.; Leng, Y. L.; Zhang, J. H.; Lin, Q.; Zhang, Y. M.; Yao, H. Sens. Actuators, B:Chem. 2017, 239, 671.
[47] Kiyose, K.; Hanaoka, K.; Oushiki, D.; Nakamura, T.; Kajimura, M.; Suematsu, M.; Nishimatsu, H.; Yamane, T.; Terai, T.; Hirata, Y.; Nagano, T. J. Am. Chem. Soc. 2010, 132, 15846.
[48] Yang, L.; Qu, W.; Zhang, X.; Hang, Y.; Hua, J. Analyst 2015, 140, 182.
[49] Liu, X.; Weinert, Z. J.; Sharafi, M.; Liao, C.; Li, J.; Schneebeli, S. T. Angew. Chem., Int. Ed. 2015, 54, 12772.
[50] Qu, Y.; Zhang, X.; Wang, L.; Yang, H.; Yang, L.; Cao, J.; Hua, J. RSC Adv. 2016, 6, 22389.
[51] Gu, P.-Y.; Wang, C.; Nie, L.; Long, G.; Zhang, Q. RSC Adv. 2016, 6, 37929.
[52] Liu, Y.; Ye, M.; Ge, Q.; Qu, X.; Guo, Q.; Hu, X.; Sun, Q. Anal. Chem. 2016, 88, 1768.
[53] Wang, L.; Liu, S.; Hao, C.; Zhang, X.; Wang, C.; He, Y. Sens. Actuators, B:Chem. 2016, 229, 145.
[54] Zhang, S. G. Biotechnol. Adv. 2002, 20, 321.
[55] Gilday, L. C.; Robinson, S. W.; Barendt, T. A.; Langton, M. J.; Mullaney, B. R.; Beer, P. D. Chem. Rev. 2015, 115, 6114.
[56] Kazuma, G.; Tetsuo, A.; Hiroyuki, I. Acta Crystallogr. 2007, 63, 17.
[57] Tu, L.; Hsin, R. C.; Hong, Y. L.; Hung, L. L. Cryst. Growth Des. 2012, 12, 5897.
[58] Tran, N. T.; Wilson, S. O.; Franz, A. K. Chem. Commun. 2014, 50, 3738.
[59] Metz, A. E.; Podlesny, E. E.; Carroll, P. J.; Klinghoffer, A. N.; Kozlowski, M. C. J. Am. Chem. Soc. 2014, 136, 10601.
[60] Nayak, A.; Pedireddi, V. R. Cryst. Growth Des. 2016, 16, 5966.
[61] Ritter, K.; Pehlken, C.; Sorsche, D.; Rau, S. Dalton Trans. 2015, 44, 8889.
[62] Hugo, V.; Macarena, P.; Eduardo, P. Inorg. Chem. 2015, 54, 3654.
[63] Mohammad, O. B.; Jeffery, D. M.; Haoran, S. Cryst. Growth Des. 2015, 15, 2235.
[64] Shuster, V.; Gambarotta, S.; Nikiforov, G. B.; Budzelaar, P. H. M. Organometallics 2013, 32, 2329.
[65] Bindewald, E.; Lorenz, R.; Hübner, O.; Brox, D.; Herten, D.-P.; Kaifer, E.; Himmel, H.-J. Dalton Trans. 2015, 44, 3467.
[66] Wei, T.; Zhang, H.; Li, W.; Qu, W.; Su, J.; Lin, Q.; Zhang, Y.; Yao, H. Chin. J. Chem. 2017, 35, 1311.
[67] Gao, Y.; Li, H.; Yin, S.; Liu, G.; Cao, L.; Li, Y.; Wang, X.; Ou, Z.; Wang, X. New J. Chem. 2014, 38, 5647.
[68] Liu, Y.; Zhong, K.; Li, Z.; Wang, Y.; Chen, T.; Lee, M.; Jin, L. Y. Polym. Chem. 2015, 6, 7395.
[69] Liang, G.; Wu, G.; Wang, H.; Su, J.; Li, H.; Lin, Q.; Zhang, Y.; Wei, T. J. Inclusion Phenom. Macrocyclic Chem. 2016, 86, 173.
[70] Lee, D. C.; Brownell, L. V.; Jang, K.; Han, S. J.; Robins, K. A. Phys. Chem. Chem. Phys. 2015, 17, 2457.
[71] Jiang, K.; Ma, S.; Bi, H.; Chen, D.; Han, X. J. Mater. Chem. A 2014, 2, 19208.
/
| 〈 |
|
〉 |