

Chinese Journal of Organic Chemistry >
Cross-Dehydrogenative Coupling Reactions Applied in the Construction of Privileged Heterocycles
Received date: 2017-03-20
Revised date: 2017-06-14
Online published: 2017-07-07
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 81325020, 81761128022).
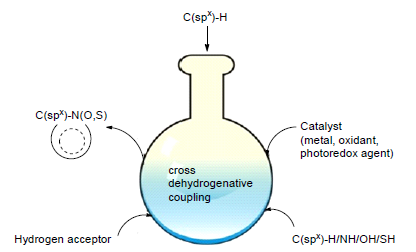
The reactions in which a new C-C bond is formed via a direct coupling of two C-H bonds are termed as cross dehydrogenative coupling (CDC). The coupling reactions would not need the pre-activation of the inert C-H bonds by any func-tional groups, featured with straightforwardness, atom-and step-economy and environmently benigness. Heterocycles are widely found in bioactive natural products and pharmaceuticals, designated as drug-like privileged scaffolds since they endowed diverse pharmacological effects. Preparation of these privileged structures by means of CDC confers distinctive advantages and enhances the discovery of lead compounds in medicinal chemistry. The application of CDC reactions in the synthesis of the heterocycles of pharmaceutical interest in recent years is summarized, focused on the construction of privileged scaffolds, such as indole, pyrrole, quinazoline, quinoxaline and the middle size and poly ring systems.
Hu Wei , Long Yaqiu . Cross-Dehydrogenative Coupling Reactions Applied in the Construction of Privileged Heterocycles[J]. Chinese Journal of Organic Chemistry, 2017 , 37(11) : 2850 -2858 . DOI: 10.6023/cjoc201703033
[1] (a) Wang, M.; Wang, Z.; Shang, M.; Dai, H. Chin. J. Org. Chem. 2015, 35, 570(in Chinese). (王明明, 王子潇, 商明, 戴辉雄, 有机化学, 2015, 35, 570.)
(b) Lu, B.; Li, X.; Lin, Y. Chin. J. Org. Chem. 2015, 35, 2275(in Chinese). (卢贝丽, 李现艳, 林咏梅, 有机化学, 2015, 35, 2275.)
(c) Ding, Z.; Tan, Q.; Liu, B.; Zhang, K.; Xu, B. Acta Chim. Sinica 2015, 73, 1302(in Chinese). (丁正伟, 谭启涛, 刘秉新, 张可, 许斌, 化学学报, 2015, 73, 1302.)
(d) Yu, J.-Q.; Ding, K.-L. Acta Chim. Sinica 2015, 73, 1223(in Chinese). (余金权, 丁奎岭, 化学学报, 2015, 73, 1223.)
[2] (a) Girard, S. A.; Knauber, T.; Li, C.-J. Angew. Chem., Int. Ed. 2014, 53, 74.
(b) Li, G.; Nakamura, H. Angew. Chem., Int. Ed. 2016, 55, 6758.
[3] (a) Li, Z.; Li, C.-J. J. Am. Chem. Soc. 2005, 127, 6968.
(b) Li, Z.; Li, C.-J. J. Am. Chem. Soc. 2006, 128, 56.
(c) Zhang, Y.; Li, C.-J. J. Am. Chem. Soc 2006, 128, 4242.
[4] (a) Zhang, C.; Jiao, N. Angew. Chem., Int. Ed. 2010, 49, 6174.
(b) Han, W.; Mayer, P.; Ofial, A. R. Angew. Chem., Int. Ed. 2011, 50, 2178.
(c) Antonchick, A. P.; Burgmann, L. Angew. Chem., Int. Ed. 2013, 52, 3267.
(d) Meng, Z.; Sun, S.; Yuan, H.; Lou, H.; Liu, L. Angew. Chem., Int. Ed. 2014, 53, 543.
(e) Zhou, L.; Xu, B.; Zhang, J. Angew. Chem., Int. Ed. 2015, 54, 9092.
[5] Gensch, T.; Klauck, F. J. R.; Glorius, F. Angew. Chem., Int. Ed. 2016, 55, 11287.
[6] Lin, J.-P.; Zhang, F.-H.; Long, Y.-Q. Org. Lett. 2014, 16, 2822.
[7] Hu, W.; Lin, J.-P.; Song, L.-R.; Long, Y.-Q. Org. Lett. 2015, 17, 1268.
[8] Zhao, M.; Wang, F.; Li, X. Org. Lett. 2012, 14, 1412.
[9] Vanjari, R.; Guntreddi, T.; Kumar, S.; Singh, K. N. Chem. Commun. 2015, 51, 366.
[10] (a) Wang, L.; Woods, K. W.; Li, Q.; Barr, K. J.; McCroskey, R. W.; Hannick, S. M.; Gherke, L.; Credo, R. B.; Hui, Y.-H.; Marsh, K.; Warner, R.; Lee, J. Y.; Zielinski-Mozng, N.; Frost, D.; Rosenberg, S. H.; Sham, H. L. J. Med. Chem. 2002, 45, 1697.
(b) Zeng, L.-F.; Wang, Y.; Kazemi, R.; Xu, S.; Xu, Z.-L.; Sanchez, T. W.; Yang, L.-M.; Debnath, B.; Odde, S.; Xie, H.; Zheng, Y.-T.; Ding, J.; Neamati, N.; Long, Y.-Q. J. Med. Chem 2012, 55, 9492.
(c) Lam, T.; Hilgers, M. T.; Cunningham, M. L.; Kwan, B. P.; Nelson, K. J.; Brown-Driver, V.; Ong, V.; Trzoss, M.; Hough, G.; Shaw, K. J.; Finn, J. J. Med. Chem. 2014, 57, 651.
[11] Li, C.-J. Acc. Chem. Res. 2009, 42, 335.
[12] Xue, D.; Long, Y.-Q. J. Org. Chem. 2014, 79, 4727.
[13] Zhu, C.; Yang, B.; Qiu, Y.; Backvall, J. E. Angew. Chem., Int. Ed. 2016, 55, 14405.
[14] (a) Cheng, Y.; Shen, J.; Peng, R.-Z.; Wang, G.-F.; Zuo, J.-P.; Long, Y.-Q. Bioorg. Med. Chem. Lett. 2016, 26, 2900.
(b) Zhi, Y.; Gao, L.-X.; Jin, Y.; Tang, C.-L.; Li, J.-Y.; Li, J.; Long, Y.-Q. Bioorg. Med. Chem. 2014, 22, 3670.
[15] (a) Shi, Z.; Glorius, F. Angew. Chem., Int. Ed. 2012, 51, 9220.
(b) Li, J.; Li, C.; Yang, S.; An, Y.; Wu, W.; Jiang, H. J. Org. Chem. 2016, 81, 7771.
(c) Gao, S.; Wu, Z.; Fang, X.; Lin, A.; Yao, H. Org. Lett. 2016, 18, 3906.
(d) Chen, J.; Wu, J. Angew. Chem., Int. Ed. 2017, 56, 3951.
(e) Li, J.; Li, C.; Yang, S.; An, Y.; Wu, W.; Jiang, H. J. Org. Chem. 2016, 81, 2875.
[16] Würtz, S.; Rakshit, S.; Neumann, J. J.; Dröge, T.; Glorius, F. Angew. Chem., Int. Ed. 2008, 120, 7340.
[17] Shi, Z.; Zhang, C.; Li, S.; Pan, D.; Ding, S.; Cui, Y.; Jiao, N. Angew. Chem., Int. Ed. 2009, 48, 4572.
[18] Yu, W.; Du, Y.; Zhao, K. Org. Lett. 2009, 11, 2417.
[19] Guan, Z.-H.; Yan, Z.-Y.; Ren, Z.-H.; Liu, X.-Y.; Liang, Y.-M. Chem. Commun. 2010, 46, 2823.
[20] Zoller, J.; Fabry, D. C.; Ronge, M. A.; Rueping, M. Angew. Chem., Int. Ed. 2014, 53, 13264.
[21] Wei, Y.; Deb, I.; Yoshikai, N. J. Am. Chem. Soc. 2012, 134, 9098.
[22] Jiang, L.; Jin, W.; Hu, W. ACS Catal. 2016, 6, 6146.
[23] Tanitame, A.; Oyamada, Y.; Ofuji, K.; Fujimoto, M.; Iwai, N.; Hiyama, Y.; Suzuki, K.; Ito, H.; Terauchi, H.; Kawasaki, M.; Nagai, K.; Wachi, M.; Yamagishi, J.-I. J. Med. Chem. 2004, 47, 3693.
[24] Wu, C.-H.; Hung, M.-S.; Song, J.-S.; Yeh, T.-K.; Chou, M.-C.; Chu, C.-M.; Jan, J.-J.; Hsieh, M.-T.; Tseng, S.-L.; Chang, C.-P.; Hsieh, W.-P.; Lin, Y.; Yeh, Y.-N.; Chung, W.-L.; Kuo, C.-W.; Lin, C.-Y.; Shy, H.-S.; Chao, Y.-S.; Shia, K.-S. J. Med. Chem. 2009, 52, 4496.
[25] Fustero, S.; Sánchez-Roselló, M.; Barrio, P.; Simón-Fuentes, A. Chem. Rev. 2011, 111, 6984.
[26] Neumann, J. J.; Suri, M.; Glorius, F. Angew. Chem., Int. Ed. 2010, 49, 7790.
[27] (a) Zhang, G.; Zhao, Y.; Ge, H. Angew. Chem., Int. Ed. 2013, 52, 2559.
(b) Wu, X.; Wang, M.; Zhang, G.; Zhao, Y.; Wang, J.; Ge, H. Chem. Sci. 2015, 6, 5882.
[28] Murarka, S.; Studer, A. Org. Lett. 2011, 13, 2746.
[29] Ye, J. T.; Ma, S. M. Acc. Chem. Res. 2014, 47, 989.
[30] Xiao, T.; Li, L.; Lin, G.; Mao, Z.-W.; Zhou, L. Org. Lett. 2014, 16, 4232.
[31] Zhang, Z.; Dai, Z.; Ma, X.; Liu, Y.; Ma, X.; Li, W.; Ma, C. Org. Chem. Front. 2016, 3, 799.
[32] Ackermann, L.; Pospech, J. Org. Lett. 2011, 13, 4153.
[33] Zhang, Z.; Xie, C.; Tan, X.; Song, G.; Wen, L.; Gao, H.; Ma, C. Org. Chem. Front. 2015, 2, 942.
[34] Sun, M.; Zhang, T.; Bao, W. J. Org. Chem. 2013, 78, 8155.
[35] (a) Dhiman, S.; Mishra, U. K.; Ramasastry, S. S. V. Angew. Chem., Int. Ed. 2016, 55, 7737.
(b) Garkhedkar, A. M.; Senadi, G. C.; Wang, J. J. Org. Lett. 2017, 19, 488.
(c) Cheng, J.; Li, W. P.; Duan, Y. Q.; Cheng, Y. X.; Yu, S. Y.; Zhu, C. J. Org. Lett. 2017, 19, 214.
[36] Gaster, E.; Vainer, Y.; Regev, A.; Narute, S.; Sudheendran, K.; Werbeloff, A.; Shalit, H.; Pappo, D. Angew. Chem., Int. Ed. 2015, 54, 4198.
/
| 〈 |
|
〉 |