

Chinese Journal of Organic Chemistry >
Research Progress of Organophosphine-Catalyzed Annulation Reaction of Electron-Deficient Alkynoates or Ynones
Received date: 2017-05-07
Revised date: 2017-06-28
Online published: 2017-07-07
Supported by
Project supported by the Committee of Science and Technology of Xinjiang (No. 2016D01A016).
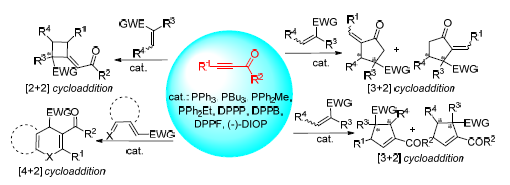
Organophosphine catalyst is a kind of strong nucleophilic Lewis base. It is widely used in the field of organic synthesis. An active and important zwitterion intermediate can be generated via nucleophilic addition of the tertiary phosphine to electron-deficient alkynoates or ynones and achieves further transformation, including isomerization, α-, β-, γ-addition reactions and[2+2],[3+2],[4+2] cycloaddition reactions. Varieties of pharmaceuticals, natural products and other bioactive moleculars could be efficiently synthesized through organophosphine-catalyzed cycloaddition reaction. The research of organ-ophosphine-catalyzed cycloaddition reaction of electron-deficient alkynoates or ynones has gained more attention. The recent development of organophosphine-catalyzed cycloaddition reaction of electron-deficient alkynoates or ynones is summarized.
Zhang Jiayong, Abudukeremu Munira, Miao Zhiwei . Research Progress of Organophosphine-Catalyzed Annulation Reaction of Electron-Deficient Alkynoates or Ynones[J]. Chinese Journal of Organic Chemistry, 2017 , 37(11) : 2859 -2872 . DOI: 10.6023/cjoc201705011
[1] For selected reviews on tertiary organophosphine-catalyzed annulation reactions of electron-deficient alkynoates or ynones, see:(a) Cowen, B. J.; Miller, S. J. Chem. Soc. Rev. 2009, 38, 3102.
(b) Ye, L. W.; Zhou, J.; Tang, Y. Chem. Soc. Rev. 2008, 37, 1140.
(c) Nair, V.; Menon, R. S.; Sreekanth, A. R.; Abhilash, N.; Biju, A. T. Acc. Chem. Res. 2006, 39, 520.
(d) Methot, J. L.; Roush, W. R. Adv. Synth. Catal. 2004, 346, 1035.
(e) Marinetti, A; Voituriez, A. Synlett 2010, 174.
(f) Fan, Y. C.; Kwon, O. Chem. Commun. 2013, 49, 11588.
(g) Wang, Z. M.; Xu, X. Z.; Kwon, O. Chem. Soc. Rev. 2014, 43, 2927.
(h) Xiao, Y.; Guo, H.; Kwon, O. Aldrichim. Acta 2016, 49, 3.
[2] (a) Lu, X. Y.; Zhang, C. M.; Xu, Z. R. Acc. Chem. Res. 2001, 34, 535.
(b) Jacobsen, M. J.; Funder, E. D.; Cramer, J. R.; Gothelf, K. V. Org. Lett. 2005, 13, 3418.
(c) Xu, Z. R.; Lu, X. Y. J. Org. Chem. 1998, 63, 5031.
(d) Meng, L. G.; Cai, P. J.; Guo, Q. X.; Xue, S. J. Org. Chem. 2008, 73, 8491.
(e) Yang, Z. L.; Yu, H.; Zhang, L.; Wei, H.; Xiao, Y. M.; Chen, L. Z.; Guo, H. C. Chem. Asian J. 2014, 9, 313.
(f) Ge, X.; Chen, X. Z.; Qian, C. Chin. J. Org. Chem. 2016, 36, 1208(in Chinese). (葛新, 陈新志, 钱超, 有机化学, 2016, 36, 1208.)
(g) Yang, L. J.; Ma, J. A. Acta Chim. Sinica 2016, 74, 130(in Chinese). (杨丽军, 马军安, 化学学报, 2016, 74, 130.)
(h) Zhou, W.; Gao, L. H.; Tao, M. N.; Su, X.; Zhao, Q. J.; Zhang, J. L. Acta Chim. Sinica 2016, 74, 800(in Chinese). (周伟, 高利华, 陶梦娜, 宿晓, 赵庆杰, 张俊良, 化学学报, 2016, 74, 800.)
[3] (a) Du, Y. S.; Yu, Y. H.; Lu, X. Y. J. Org. Chem. 2002, 67, 8901.
(b) Zhao, G. L.; Shi, M. J. Org. Chem. 2005, 70, 9975.
(c) Sampath, M.; Loh, T. P. Chem. Sci. 2010, 1, 739.
(d) Mbofana, C. T.; Miller, S. J. ACS Catal. 2014, 4, 3671.
(e) Lian, Z.; Shi, M. Eur. J. Org. Chem. 2012, 581.
(f) Kuroda, H.; Tomita, L.; Endo, T. Org. Lett. 2003, 5, 129.
(g) Ramachary, D. B.; Venkaiah, C.; Madhavachary, R. Org. Lett. 2013, 15, 3042.
(h) Wei, Y.; Shi, M. Chem.-Asian J. 2014, 9, 2720.
(i) Wang, S. X.; Han, X. Y.; Zhong, F. R.; Wang, Y. Q.; Lu, Y. X. Synlett 2011, 19, 2766.
(j) Zhao, Q. Y.; Lian, Z.; Wei, Y.; Shi, M. Chem. Commun. 2012, 48, 1724.
(k) Wang, Z. M.; Xu, X. Z.; Kwon, O. Chem. Soc. Rev. 2014, 43, 2927.
(l) Xie, P. Z.; Huang, Y. Org. Biomol. Chem. 2015, 13, 8578.
(m) Li, W. B.; Zhang, J. L. Chem. Soc. Rev. 2016, 45, 1657.
(n) Wang, T. L.; Han, X. Y.; Zhong, F. R.; Yao, W. J.; Lu, Y. X. Acc. Chem. Res. 2016, 49, 1369.
(o) Xiao, Y. M.; Sun, Z. H.; Guo, H. C.; Kwon, O. Beilstein J. Org. Chem. 2014, 10, 2089.
[4] Zhang, C. M.; Lu, X. Y. J. Org. Chem. 1995, 60, 2906.
[5] Xu, Z. R.; Lu, X. Y. Tetrahedron lett. 1999, 40, 549.
[6] Du, Y. S.; Lu, X. Y. J. Org. Chem. 2003, 68, 6463.
[7] Pham, T. Q.; Pyne, S. G.; Skelton, B. W.; White, A. H. J. Org. Chem. 2005, 70, 6369.
[8] Zhang, X. C.; Cao, S. H.; Wei, Y.; Shi, M. Org. Lett. 2011, 13, 1142.
[9] Sampath, M. S.; Loh, T. P. Chem. Sci. 2010, 1, 739.
[10] Pinto, N.; Neel, M.; Panossian, A.; Retailleau, P.; Frison, G.; Voituriez, A.; Marinetti, A. Chem.-Eur. J. 2010, 16, 1033.
[11] Wang, J. C.; Krische, M. J. Angew. Chem., Int. Ed. 2003, 42, 5855.
[12] Mbofana, C. T.; Miller, S. J. ACS Catal. 2014, 4, 3671.
[13] Gong, H.; Sun, J.; Yan, C. G. Synthesis 2014, 2327.
[14] Zhang, X. Y.; Shen, Z.; Hu, L. L.; Wang, L. J.; Lin, Y. S.; Xie, J. W.; Cui, H. L. Tetrahedron Lett. 2016, 57, 3790.
[15] Zhang, J. Y.; Zhang, M. X.; Li, Y. M.; Liu, S., Miao, Z. W. RSC Adv. 2016, 6, 107984.
[16] Nozaki, K.; Sato, N.; Ikeda, K.; Takaya, H. J. Org. Chem. 1996, 61, 4516.
[17] Sampath, M.; Lee, P. Y. B.; Loh. T. P. Chem. Sci. 2011, 2, 1988.
[18] Du, D.; Jiang, Y.; Xu, Q.; Shi, M. Adv. Synth. Catal. 2013, 355, 2249.
[19] Huang, Z. S.; Chen, Q. Q.; Yang, X. Q.; Liu, Y.; Zhang, L.; Liu, T.; Zhou, Q. F. Org. Chem. Front. 2017, 4, 967.
[20] Li, J. H.; Du, D. M. Adv. Synth. Catal. 2015, 357, 3986.
[21] Khong, S.; Kwon, O. J. Org. Chem. 2012, 77, 8257.
[22] Liou, K. F.; Cheng. C. H. J. Chem. Soc., Chem. Commun., 1995, 2473.
[23] Wilson, J. E.; Sun, J.; Fu, G. C. Angew. Chem., Int. Ed. 2010, 49, 161.
[24] Pinto, N.; Neel, M.; Panossian, A.; Retailleau, P.; Frison, G.; Voituriez, A.; Marinetti. A. Chem.-Eur. J. 2010, 16, 1033.
[25] Ramachary, D. B.; Venkaiah, C.; Krishna, P. M. Org. Lett. 2013, 15, 4714.
[26] Liang, L.; Li, E. Q.; Xie, P. Z.; Huang, Y. Chem.-Asian J. 2014, 9, 1270.
[27] Kuroda, H.; Tomita, I.; Endo, T. Org. Lett. 2003, 5, 129.
[28] Yang, L. H.; Xie, P. Z.; Li, E. Q.; Li, X.; Huang, Y.; Chen, R. Y. Org. Biomol. Chem. 2012, 10, 7628.
[29] Chen, Q. Q.; Li, K. X.; Lu, T.; Zhou, Q. F. RSC Adv. 2016, 6, 24792.
[30] Ramachray, D. B.; Krishna, P. M.; Reddy, T. P. Org. Biomol. Chem. 2016, 14, 6413.
[31] Lian, Z.; Wei, Y.; Shi, M. Tetrahedron 2012, 68, 2401.
[32] Lian, Z.; Shi. M. Eur. J. Org. Chem. 2012, 581.
[33] Lian, Z.; Shi, M. Org. Biomol. Chem., 2012, 10, 8048.
[34] Lian, Z.; Xu, Q.; Shi, M. Adv. Synth. Catal. 2013, 355, 3344.
[35] Li, Z.; Yu, H.; Liu, Y.; Zhou, L. J.; Sun, Z. H.; Guo, H. C. Adv. Synth. Catal. 2016, 358, 1880.
[36] Zhu, C. Z.; Sun, Y. L.; Wei, Y.; Shi, Min. Adv. Synth. Catal. 2017, 359, 1263.
/
| 〈 |
|
〉 |