

Chinese Journal of Organic Chemistry >
Asymmetric Friedel-Crafts Alkylation of Pyrrole with Chalcones Catalyzed by a Dinuclear Zinc Catalyst
Received date: 2017-06-19
Revised date: 2017-07-15
Online published: 2017-08-09
Supported by
Project supported by the National Natural Science Foundation of China (No. 21272216) and the Education Department of Henan Province (Nos. 17B150014, 18B150028).
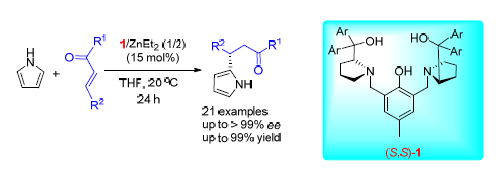
An intramolecular dinuclear zinc complex was used in asymmetric Friedel-Crafts alkylation of pyrrole with a wide range of chalcone derivatives. This dinuclear zinc complex was prepared in situ by reacting the chiral ligand (S,S)-1 with 2 equiv. of ZnEt2. A series of β-pyrrole-substituted dihydrochalcones were formed mostly in excellent yields (up to 99%) and excellent enantioselectivities (up to >99% ee) by using 15 mol% catalyst loading under mild conditions. A possible mechanism was proposed to explain the origin of the asymmetric induction.
Hua Yuanzhao , Han Xingwang , Huang Lihua , Wang Mincan . Asymmetric Friedel-Crafts Alkylation of Pyrrole with Chalcones Catalyzed by a Dinuclear Zinc Catalyst[J]. Chinese Journal of Organic Chemistry, 2018 , 38(1) : 237 -245 . DOI: 10.6023/cjoc201706027
[1] (a) Matsunaga, S.; Ohshima, T.; Shibasaki, M. Adv. Synth. Catal. 2002, 344, 3.
(b) Ma, J. A.; Cahard, D. Angew. Chem., Int. Ed. 2004, 43, 4566.
(c) Shibasaki, M.; Kanai, M.; Matsunaga, S.; Kumagai, N. Acc. Chem. Res. 2009, 42, 1117.
(d) Trost, B. M.; Bartlett, M. J. Acc. Chem. Res. 2015, 48, 688.
[2] (a) Trost, B. M.; Ito, H. J. Am. Chem. Soc. 2000, 122, 12003.
(b) Trost, B. M.; Fettes, A. J. Am. Chem. Soc. 2004, 126, 2660.
(c) Trost, B. M.; Shin, S. J. Am. Chem. Soc. 2005, 127, 8602.
[3] (a) Trost, B. M.; Yeh, V. S. C. Angew. Chem., Int. Ed. 2002, 41, 861.
(b) Trost, B. M.; Lupton, D. W. Org. Lett. 2007, 9, 2023.
[4] (a) Trost, B. M.; Terrell, L. R. J. Am. Chem. Soc. 2003, 125, 338.
(b) Zhao, D.; Wang, L.; Yang, D.; Zhang, Y.; Wang, R. Angew. Chem., Int. Ed. 2012, 51, 7523.
(c) Trost, B. M.; Hung, C.-I. J. Am. Chem. Soc. 2015, 137, 15940.
(d) Wang, X.-W.; Hua, Y.-Z.; Wang, M.-C. J. Org. Chem. 2016, 81, 9227.
[5] (a) Trost, B. M.; Mino, T. J. Am. Chem. Soc. 2003, 125, 2410.
(b) Trost, B. M.; Malhotra, S.; Mino, T.; Rajapaksa, N. S. Chem. Eur. J. 2008, 14, 7648.
[6] (a) Trost, B. M.; Hisaindee, S. Org. Lett. 2006, 8, 6003.
(b) Trost, B. M.; Hitce, J. J. Am. Chem. Soc. 2009, 131, 4572
(c) Trost, B. M.; Hirano, K. Angew. Chem., Int. Ed. 2012, 51, 6480.
(d) Song, X.; Liu, J.; Liu, M.-M.; Wang, X.; Zhang, Z.-F.; Wang, M.-C.; Chang, J. Tetrahedron 2014, 70, 5468.
[7] (a) Trost, B. M.; Weiss, A. H.; Wangelin, A. J. J. Am. Chem. Soc. 2006, 128, 8.
(b) Trost, B. M.; Quintard, A. Angew. Chem., Int. Ed. 2012, 51, 6704.
[8] (a) Xiao, Y.; Wang, Z.; Ding, K. Chem. Eur. J. 2005, 11, 3668.
(b) Xiao, Y.; Wang, Z.; Ding, K. Macromolecules 2006, 39, 128.
[9] Trost, B. M.; Muller, C. J. Am. Chem. Soc. 2008, 130, 2438.
[10] (a) Trost, B. M.; Yeh, V. S. C. Org. Lett. 2002, 4, 3513.
(b) Trost, B. M.; Quintard, A. Org. Lett. 2012, 14, 4698.
[11] Friedel, C.; Crafts, J. M. C. R. Hebd. Seances Acad. Sci. 1877, 84, 1392.
[12] Olah, G. A.; Krishnamurti, R.; Prakash, G. K. S. In Comprehensive Organic Synthesis, Eds.:Trost, B. M.; Fleming, I., Pergamon Press, Oxford, 1991, vol. 3, p 293.
[13] (a) Bandini, M.; Melloni, A.; Umani-Ronchi, A. Angew. Chem., Int. Ed. 2004, 43, 550.
(b) Poulsen, T. B.; Jørgensen, K. A. Chem. Rev. 2008, 108, 2903.
(c) You, S.-L.; Cai, Q.; Zeng, M. Chem. Soc. Rev. 2009, 38, 2190.
(e) Lu, H.-H.; Tan, F.; Xiao, W.-J. Curr. Org. Chem. 2011, 15, 4022.
[14] Liang, X.-R.; Fan, J.-Y.; Shi, F.; Su, W. K. Tetrahedron Lett. 2010, 51, 2505.
[15] (a) Wang, W.-T.; Liu, X.-H.; Cao, W.-D.; Wang, J.; Lin, L.-L.; Feng, X.-M. Chem.-Eur. J. 2010, 16, 1664.
(b) Hua, Y.-Z.; Han, X.-W.; Yang, X.-C.; Song, X.; Wang, M.-C.; Chang, J.-B. J. Org. Chem. 2014, 79, 11690.
[16] (a) Palomo, C.; Oiarbide, M.; Kardak, B. G.; Garcia, J. M.; Linden, A. J. Am. Chem. Soc. 2005, 127, 4154.
(b) Liu, L.; Ma, H.; Xiao, Y.; Du, F.; Qin, Z.; Li, N.; Fu, B. Chem. Commun. 2012, 48, 9281.
[17] (a) Zeng, M.; You, S.-L. Synlett 2010, 1289.
(b) Lancianesi, S.; Palmieri, A.; Petrini, M. Chem. Rev. 2014, 114, 7108.
(c) Yin, B.; Wu, Y.; Ma, H.; Ma, X.; Fu, B.; Liu, J. Chin. J. Org. Chem. 2015, 35, 2119(in Chinese).(殷伯翰, 吴燕华, 麻红利, 马晓东, 傅滨, 刘吉平, 有机化学, 2015, 35, 2119.)
[18] Zhuo, C.-X.; Zhou, Y.; You, S.-L. J. Am. Chem. Soc. 2014, 136, 6590.
[19] (a) Wang, M.-C.; Zhang, Q.-J.; Zhao, W.-X.; Wang, X.-D.; Ding, X.; Jing, T.-T.; Song, M.-P. J. Org. Chem. 2008, 73, 168.
(b) Hua, Y.-Z.; Lu, L.-J.; Huang, P.-J.; Wei, D.-H.; Tang, M.-S.; Wang, M.-C.; Chang, J.-B. Chem.-Eur. J. 2014, 20, 12394.
(c) Hua, Y.-Z.; Yang, X.-C.; Liu, M.-M.; Song, X.; Wang, M.-C.; Chang, J.-B. Macromolecules 2015, 48, 1651.
(d) Hua, Y.-Z.; Liu, M.-M.; Huang, P.-J.; Song, X.; Wang, M.-C.; Chang, J.-B. Chem.-Eur. J. 2015, 21, 11994.
[20] (a) Bao, H.; Wu, J.; Li, H.; Wang, Z.; You, T.; Ding, K. Eur. J. Org. Chem. 2010, 6722.
(b) Bao, H.; Wang, Z.; You, T.; Ding, K. Chin. J. Chem. 2013, 31, 67.
[21] Therkelsen, F. D.; Hansen, A. L. L.; Pedersen, E. B.; Nielsen, C. Org. Biomol. Chem. 2003, 1, 2908.
/
| 〈 |
|
〉 |