

Chinese Journal of Organic Chemistry >
Progress in Iridium-Catalyzed Asymmetric Allylic Substitution Reactions with Allylic Esters
Received date: 2017-04-19
Revised date: 2017-06-21
Online published: 2017-08-11
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21172081, 21372090), the Natural Science Foundation of Guangdong Province (No. S2013020013091) and the Science and Technology Plan Projects of Guangzhou City (No. 201510010054).
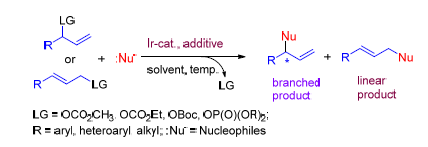
Iridium-catalyzed asymmetric allylic substitution reaction is one of the most important methods for the synthesis of chiral compounds. The recent research progress in iridium-catalyzed asymmetric allylic substitution reactions of allylic ester and its derivatives is reviewed with focus on the influences of the iridium catalysts, the substrate structures of allylic ester and its derivatives, the type of nucleophiles, the effects of solvents and additives on asymmetric substitution reaction. Moreover, the possible mechanisms are also discussed in this review.
Deng Yingying , Yang Wen , Yang Xin , Yang Dingqiao . Progress in Iridium-Catalyzed Asymmetric Allylic Substitution Reactions with Allylic Esters[J]. Chinese Journal of Organic Chemistry, 2017 , 37(12) : 3039 -3059 . DOI: 10.6023/cjoc201704034
[1] (a) Liu, Z.-Q.; Du, H.-F. Org. Lett. 2010, 12, 3054.
(b) Zhang, P.; Le, H.; Kyne, R. E.; Morken, J. P. J. Am. Chem. Soc. 2011, 133, 9716.
(c) Suetsugu, S.; Nishiguchi, H.; Tsukano, C.; Takemoto, Y. Org. Lett. 2014, 16, 996.
(d) Katcher, M. H.; Norrby, P. O.; Doyle, A. G. Organometallics 2014, 33, 2121.
[2] (a) Hughes, D. L.; Lloyd-Jones, G. C.; Krska, S. W.; Gouriou, L.; Bonnet, V. D.; Jack, K.; Sun, Y.-K.; David, J. M.; Reamer, R. A. Proc. Natl. Acad. Sci. U. S. A. 2004, 101, 5379.
(b) Litto, R. D.; Benessere, V.; Ruffo, F.; Moberg, C. Eur. J. Org. Chem. 2009, 1352.
[3] Moberg, C. Top Organomet. Chem. 2012, 38, 209.
[4] (a) Jegelka, M.; Plietker, B. Org. Lett. 2009, 11, 3462.
(b) Jegelka, M.; Plietker, B. Chem. Eur. J. 2011, 17, 10417.
[5] (a) Trost, B. M.; Rao, M.; Dieskau, A. P. J. Am. Chem. Soc. 2013, 135, 18697.
(b) Kawatsura, M.; Uchida, K.; Terasaki, S.; Tsuji, H.; Minakawa, M.; Itoh, T. Org. Lett. 2014, 16, 1470.
[6] Tan, Z.-Z.; Wan, X.-L.; Zang, Z.-H.; Qian, Q.; Deng, W.; Gong, H.-G. Chem. Commun. 2014, 50, 3827.
[7] (a) Vrieze, D. C.; Hoge, G. S.; Hoerter, P. Z.; Van Haitsma, J. T.; Samas, B. M. Org. Lett. 2009, 11, 3140.
(b) Arnold, J. S.; Nguyen, H. M. J. Am. Chem. Soc. 2012, 134, 8380.
[8] (a) Yang, S.-C.; Feng, W.-H.; Gan, K.-H. Tetrahedron 2006, 62, 3752.
(b) Zhang, M.; Watanabe, K. J.; Tsukamoto, M.; Shibuya, R.; Morimoto, H.; Ohshima, T. Chem.-Eur. J. 2015, 21, 1.
[9] Guduguntla, S.; Hornillos, V.; Tessier, R.; Fannanaas-Mastral, M.; Feringa, B. L. Org. Lett. 2016, 18, 252.
[10] Tosatti, P.; Nelson, A.; Marsden, S. P. Org. Biomol. Chem. 2012, 10, 3147.
[11] Zhuo, C.-X.; Zheng, C.; You, S.-L. Acc. Chem. Res. 2014, 47, 2558.
[12] Hethcox, J. C.; Shockley, S. E.; Stoltz, B. M. ACS Catal. 2016, 6, 6207.
[13] Takeuchi, R.; Kashio, M. Angew. Chem., Int. Ed. 1997, 36, 263.
[14] Janssen, J. P.; Helmchen, G. Tetrahedron Lett. 1997, 38, 8025.
[15] Butt, N. A.; Zhang, W.-B. Chem. Soc. Rev. 2015, 44, 7929.
[16] Helmchen, G.; Dahnz, A.; Dubon, P.; Schelwies, M.; Weihofen, R. Chem. Commun. 2007, 675.
[17] Wu, Y.-J.; Long, Y.-H.; Yang, D.-Q. Chin. J. Org. Chem. 2009, 29, 1522(in Chinese). (吴钰娟, 龙玉华, 杨定乔, 有机化学, 2010, 29, 1522.)
[18] Giacomina, F.; Riat, D.; Alexakis, A. Org. Lett. 2010, 12, 1156.
[19] Stanley, L. M.; Bai, C.; Ueda, M.; Hartwig, J. F. J. Am. Chem. Soc. 2010, 132, 8918.
[20] Zhao, Z.-L.; Gu, Q.; Wu, X.-Y.; You, S.-L. Chin. Chem. Lett. 2016, 27, 619.
[21] Bondzic, B. P.; Farwick, A.; Liebich, J.; Eilbracht, P. Org. Biomol. Chem. 2008, 6, 3723.
[22] Liu, W.-B.; Zheng, S.-C.; He, H.; Zhao, X.-M.; Dai, L.-X.; You, S.-L. Chem. Commun. 2009, 6604.
[23] Zhang, H.-B.; Chen, J.-T.; Zhao, X.-M. Org. Biomol. Chem. 2016, 14, 7183.
[24] Liu, W.-B.; Zheng, C.; Zhuo, C.-X.; Dai, L.-X.; You, S.-L. J. Am. Chem. Soc. 2012, 134, 4812.
[25] Xu, Q.-L.; Dai, L.-X.; You, S.-L. Adv. Synth. Catal. 2012, 354, 2275.
[26] Zhan, M.; Li, R.-Z.; Mou, Z.-D.; Cao, C.-G.; Liu, J.; Chen, Y.-W.; Niu, D.-W. ACS Catal. 2016, 6, 3381.
[27] (a) Trost, B. M.; Jiang, C. H. Synthesis 2006, 369.
(b) Behenna, D. C.; Stoltz, B. M. Top Organomet. Chem. 2013, 44, 281.
[28] Chen, W.-Y.; Hartwig, J. F. J. Am. Chem. Soc. 2013, 135, 2068.
[29] Chen, W.-Y.; Hartwig, J. F. J. Am. Chem. Soc. 2014, 136, 377.
[30] Liu, W.-B.; Reeves, C. M.; Virgil, S. C.; Stoltz, B. M. J. Am. Chem. Soc. 2013, 135, 10626.
[31] Liu, W.-B.; Reeves, C. M.; Stoltz, B. M. J. Am. Chem. Soc. 2013, 135, 17298.
[32] Liu, W.-B.; Okamoto, N.; Alexy, E. J.; Hong, A. Y.; Tran, K.; Stoltz, B. M. J. Am. Chem. Soc. 2016, 138, 5234.
[33] Liu, J.; Cao, C.-G.; Sun, H.-B.; Zhang, X.; Niu, D.-W. J. Am. Chem. Soc. 2016, 138, 13103.
[34] (a) Krautwald, S.; Schafroth, M. A.; Sarlah, D.; Carreira, E. M. J. Am. Chem. Soc. 2014, 136, 3020.
(b) Sandmeier, T.; Krautwald, S.; Zipfel, H. F.; Carreira, E. M. Angew. Chem., Int. Ed. 2015, 54, 14363.
[35] (a) Yao, K.; Liu, D.-L.; Yuan, Q.-J.; Imamoto, T.; Liu, Y.-G.; Zhang, W.-B. Org. Lett. 2016, 18, 6296.
(b) Huo, X.-H.; Yang, G.-Q.; Liu, D.-L.; Liu, Y.-G.; Gridnev, I. D.; Zhang, W.-B. Angew. Chem., Int. Ed. 2014, 53, 6776.
[36] Krautwald, S.; Sarlah, D.; Schafroth, M. A.; Carreira, E. M. Science 2013, 340, 1065.
[37] Wei, X.; Liu, D.-L.; An, Q.-J.; Zhang, W.-B. Org. Lett. 2015, 17, 5768.
[38] Liu, W.-B.; He, H.; Dai, L.-X.; You, S.-L. Org. Lett. 2008, 10, 1815.
[39] Zhou, C.-X.; Wu, Q.-F.; Zhou, Q.; Xu, Q.-L.; You, S.-L. J. Am. Chem. Soc. 2013, 135, 8169.
[40] Zhuo, C.-X.; Cheng, Q.; Liu, W.-B.; Zhao, Q.; You, S.-L. Angew. Chem., Int. Ed. 2015, 54, 8475.
[41] Wu, Q.-F.; Liu, W.-B.; Zhuo, C.-X.; Rong, Z.-Q.; Ye, K.-Y.; You, S.-L. Angew. Chem., Int. Ed. 2011, 50, 4455.
[42] Xu, Q.-L.; Dai, L.-X.; You, S.-L. Org. Lett. 2012, 14, 2579.
[43] Cheng, Q.; Wang, Y.; You, S.-L. Angew. Chem., Int. Ed. 2016, 55, 3496.
[44] Chen, W.-Y.; Hartwig, J. F. J. Am. Chem. Soc. 2012, 134, 15249.
[45] Chen, W.-Y.; Chen, M.; Hartwig, J. F. J. Am. Chem. Soc. 2014, 136, 15825.
[46] Chen, M.; Hartwig, J. F. Angew. Chem., Int. Ed. 2014, 53, 8691.
[47] Chen, M.; Hartwig, J. F. J. Am. Chem. Soc. 2015, 137, 13972.
[48] Chen, M.:Hartwig, J. F. Angew. Chem., Int. Ed. 2014, 53, 12172.
[49] Chen, M.; Hartwig, J. F. Angew. Chem., Int. Ed. 2016, 55, 11651.
[50] Jiang, X.-Y.; Chen, W.-Y.; Hartwig, J. F. Angew. Chem., Int. Ed. 2016, 55, 5819.
[51] Alexakis, A.; Hajjaji, S. E.; Polet, D.; Rathgeb, X. Org. Lett. 2007, 9, 3393.
[52] Polet, D.; Rathgeb, X.; Falciola, C. A.; Langlois, J. B.; Hajjaji, S. E.; Alexakis, A. Chem. Eur. J. 2009, 15, 1205.
[53] Hamilton, J. Y.; Sarlah, D.; Carreira, E. M. Angew. Chem., Int. Ed. 2015, 54, 7644.
[54] Liu, X.-J.; You, S.-L. Angew. Chem., Int. Ed. 2017, 56, 4002.
[55] Breitler, S.; Carreira, E. M. J. Am. Chem. Soc. 2015, 137, 5296.
[56] Pouy, M. J.; Leitner, A.; Weix, D. J.; Ueno, S.; Hartwig, J. F. Org. Lett. 2007, 9, 3949.
[57] Pouy, M. J.; Stanley, L. M.; Hartwig, J. F. J. Am. Chem. Soc. 2009, 131, 11312.
[58] Weihofen, R.; Tverskoy, O.; Helmchen, G. Angew. Chem., Int. Ed. 2006, 45, 5546.
[59] Spiess, S.; Berthold, C.; Weihofen, R.; Helmchen, G. Org. Biomol. Chem. 2007, 5, 2357.
[60] Weix, D. J.; Markovic, D.; Ueda, M.; Hartwig, J. F. Org. Lett. 2009, 11, 2944.
[61] Markovic, D.; Hartwig, J. F. J. Am. Chem. Soc. 2007, 129, 11680.
[62] Ye, K.-Y.; Dai, L.-X.; You, S.-L. Org. Biomol. Chem. 2012, 10, 5932.
[63] Ye, K.-Y.; Dai, L.-X.; You, S.-L. Chem. Eur. J. 2014, 20, 3040.
[64] Stanley, L. M.; Hartwig, J. F. J. Am. Chem. Soc. 2009, 131, 8971.
[65] Liu, W.-B.; Zhang, X.; Dai, L.-X.; You, S.-L. Angew. Chem., Int. Ed. 2012, 51, 5183.
[66] Yang, Z.-P.; Wu, Q.-F.; You, S.-L. Angew. Chem., Int. Ed. 2014, 53, 6986.
[67] Zhang, X.; Yang, Z.-P.; Huang, L.; You, S.-L. Angew. Chem., Int. Ed. 2015, 54, 1873
[68] Yang, Z.-P.; Wu, Q.-F.; Shao, W.; You, S.-L. J. Am. Chem. Soc. 2015, 137, 15899.
[69] Zhuo, C.-X.; Zhang, X.; You, S.-L. ACS Catal. 2016, 6, 5307.
[70] Ye, K.-Y.; Cheng, Q.; Zhuo, C.-X.; Dai, L.-X.; You, S.-L. Angew. Chem., Int. Ed. 2016, 55, 8113.
[71] Yang, Z.-P.; Zheng, C.; Huang, L.; Qian, C.; You, S.-L. Angew. Chem., Int. Ed. 2017, 56, 1530.
[72] Miyabe, H.; Yoshida, K.; Reddy, V. K.; Takemoto, Y. J. Org. Chem. 2009, 74, 305.
[73] Gärtner, M.; Jäkel, M.; Achatz, M.; Sonnenschein, C.; Tverskoy, O.; Helmchen, G. Org. Lett. 2011, 13, 2810.
[74] Lee, J.-H.; Lee, S.-G. Chem. Sci. 2013, 4, 2922.
[75] Satyanarayana, G.; Helmchen, G. Eur. J. Org. Chem. 2014, 2242.
[76] Grange, R. L.; Clizbe, E. A.; Counsell, E. J.; Evans, A. P. Chem. Sci. 2015, 6, 777.
[77] Kimura, M.; Uozumi, Y. J. Org. Chem. 2007, 72, 707.
[78] He, H.; Ye, K.-Y.; Wu, Q.-F.; Dai, L.-X.; You, S.-L. Adv. Synth. Catal. 2012, 354, 1084.
[79] Qu, J.-P.; Roβberg, L.; Helmchen, G. J. Am. Chem. Soc. 2014, 136, 1272.
[80] Zhao, D.-P.; Martin, F. M.; Chang, M.-C.; Otten, E.; Feringa, B. L. Chem. Sci. 2014, 5, 4216.
[81] Zheng, S.-C.; Zhang, M.; Zhao. X.-M. Chem. Eur. J. 2014, 20, 1.
[82] Zhang, M.; Zheng, S.-C.; Zhao, X.-M. Chem. Commun. 2014, 50, 4455.
[83] Gärtner, M.; Mader, S.; Seehafer, K.; Helmchen, G. J. Am. Chem. Soc. 2011, 133, 2072.
[84] Xu, Q.-L.; Dai, L.-X.; You, S.-L. Org. Lett. 2010, 12, 800.
[85] Ueda, M.; Hartwing, J. F. Org. Lett. 2010, 12, 92.
[86] Liu, W.; Zhao, X.-M.; Zhang, H.-B.; Zhang, L.; Zhao, M.-Z. Chem. Eur. J. 2014, 20, 16873.
[87] Zheng, S.-C.; Gao, N.; Liu, W.; Liu, D.-G.; Zhao, X.-M.; Cohen, T. Org. Lett. 2010, 12, 4454.
[88] Zheng, S.-C.; Huang, W.-Q.; Gao, N.; Cui, R.-M.; Zhang, M.; Zhao, X.-M. Chem. Commum. 2011, 47, 6969.
[89] Huang, W.-Q.; Zheng, S.-C.; Tang, J.-L.; Zhao, X.-M. Org. Biomol. Chem. 2011, 9, 7897.
[90] Gao, N.; Zhao, X.-M. Eur. J. Org. Chem. 2013, 2708.
[91] Topczewski, J. J.; Tewson, T. J.; Nguyen, H. M. J. Am. Chem. Soc. 2011, 133, 19318.
[92] Zhang, Q.; Stockdale, D. P.; Mixdorf, J. C.; Topczewski, J. J.; Nguyen, H. M. J. Am. Chem. Soc. 2015, 137, 11912.
/
| 〈 |
|
〉 |