

Chinese Journal of Organic Chemistry >
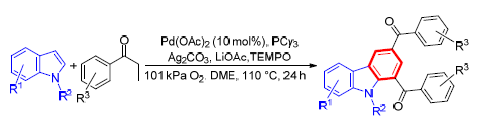
Construction of Carbazoles by Palladium-Catalyzed Direct Cross-Coupling of Indoles with in situ Generated Aryl Vinyl Ketones
Received date: 2017-05-08
Revised date: 2017-07-06
Online published: 2017-08-11
Supported by
Project supported by the National Natural Science Foundation of China (Nos.21431008,21332001,21602221,u1505242).
The synthesis of carbazoles via Pd-catalyzed direct cross-coupling of indoles with in situ generated aryl vinyl ketones by using statured ketones as the olefins source is described. This protocol obviates the need for additional preparation steps of aryl vinyl ketones and therefore opens up a new door to synthesis of carbazoles in an atom-and step-economical fashion.
Zhou Quanlong , Zhu Changlei , Wu Ge , Zhang Yuanfei , Zhang Min , Su Weiping . Construction of Carbazoles by Palladium-Catalyzed Direct Cross-Coupling of Indoles with in situ Generated Aryl Vinyl Ketones[J]. Chinese Journal of Organic Chemistry, 2017 , 37(10) : 2655 -2662 . DOI: 10.6023/cjoc201705014
[1] (a) Zhang, F.-F.; Gan, L.-L.; Zhou, C.-H. Bioorg. Med. Chem. Lett. 2010, 20, 1881.
(b) Blunt, J. W.; Copp, B. R.; Munro, M. H. G.; Northcote, P. T.; Prinsep, M. R. Nat. Prod. Rep. 2003, 20, 1.
(c) Deslandes, S.; Chassaing, S.; Delfourne, E. Mar. Drugs 2009, 7, 754.
(d) Maneerat, W.; Ritthiwigrom, T.; Cheenpracha, S.; Promgool, T.; Yossathera, K.; Deachathai, S.; Phakhodee, W.; Laphookhieo, S. J. Nat. Prod. 2012, 75, 741.
[2] (a) Roy, J.; Jana, A. K.; Mal, D. Tetrahedron 2012, 68, 6099.
(b) Knölker, H.-J.; Reddy, K. R. Chem. Rev. 2002, 102, 4303.
(c) Schmidt, A. W.; Reddy, K. R.; Knölker, H.-J. Chem. Rev. 2012, 112, 3193.
[3] (a) Ackermann, L.; Vicente, R.; Kapdi, A. R. Angew. Chem., Int. Ed. 2009, 48, 9792.
(b) Lyons, T. W.; Sanford, M. S. Chem. Rev. 2010, 110, 1147.
(c) Liu, C.; Zhang, H.; Shi, W.; Lei, A. Chem. Rev. 2011, 111, 1780.
(d) Yeung, C. S.; Dong, V. M. Chem. Rev. 2011, 111, 1215.
[4] Zhao, J.; Larock, R. C. J. Org. Chem. 2006, 71, 5340.
[5] (a) Tsang, W. C. P.; Zheng, N.; Buchwald, S. L. J. Am. Chem. Soc. 2005, 127, 14560.
(b) Jordan-Hore, J. A.; Johansson, C. C.; Beck, E. M.; Gaunt, M. J. J. Am. Chem. Soc. 2008, 130, 16184.
(c) Cho, S. H.; Yoon, J.; Chang, S. J. Am. Chem. Soc. 2011, 133, 5996.
(d) Takamatsu, K.; Hirano, K.; Satoh, T.; Miura, M. Org. Lett. 2014, 16, 2892.
[6] (a) Yamashita, M.; Horiguchi, H.; Hirano, K.; Satoh, T.; Miura, M. J. Org. Chem. 2009, 74, 7481.
(b) Jia, J.; Shi, J.; Zhou, J.; Liu, X.; Song, Y.; Xu, H. E.; Yi, W. Chem. Commun. 2015, 51, 2925.
[7] (a) Ozaki, K.; Zhang, H.; Ito, H.; Lei, A.; Itami, K. Chem. Sci. 2013, 4, 3416.
(b) Verma, A. K.; Danodia, A. K.; Saunthwal, R. K.; Patel, M.; Choudhary, D. Org. Lett. 2015, 17, 3658.
(c) Laha, J. K.; Dayal, N. Org. Lett 2015, 17, 4742.
(d) Chen, S.; Li, Y.; Ni, P.; Huang, H.; Deng, G. J. Org. Lett 2016.
[8] Grimster, N. P.; Gauntlett, C.; Godfrey, C. R.; Gaunt, M. J. Angew. Chem., Int. Ed. 2005, 44, 3125.
[9] Guo, T.; Jiang, Q.; Huang, F.; Chen, J.; Yu, Z. Org. Chem. Front. 2014, 1, 707.
[10] (a) Muzart, J. Eur. J. Org. Chem. 2010, 3779.
(b) Newhouse, T.; Turlik, A.; Chen, Y. Synlett 2016, 27, 331.
[11] (a) Nicolaou, K. C.; Gray, D. L. F.; Montagnon, T.; Harrison, S. T. Angew. Chem., Int. Ed. 2002, 41, 996.
(b) Nicolaou, K. C.; Montagnon, T.; Baran, P. S. Angew. Chem., Int. Ed. 2002, 41, 993.
(c) Nicolaou, K. C.; Montagnon, T.; Baran, P. S.; Zhong, Y. L. J. Am. Chem. Soc. 2002, 124, 2245.
(d) Uyanik, M.; Akakura, M.; Ishihara, K. J. Am. Chem. Soc. 2009, 131, 251.
(e) Nicolaou, K. C.; Zhong, Y. L.; Baran, P. S. J. Am. Chem. Soc. 2000, 122, 7596.
[12] (a) Bhattacharya, A.; DiMichele, L. M.; Dolling, U. H.; Douglas, A. W.; Grabowski, E. J. J. J. Am. Chem. Soc. 1988, 110, 3318.
(b) Walker, D.; Hiebert, J. D. Chem. Rev. 1967, 67, 153.
[13] (a) Diao, T.; Stahl, S. S. J. Am. Chem. Soc. 2011, 133, 14566.
(b) Gao, W. M.; He, Z. Q.; Qian, Y.; Zhao, J.; Huang, Y. Chem. Sci. 2012, 3, 883.
(c) Diao, T.; Wadzinski, T. J.; Stahl, S. S. Chem. Sci. 2012, 3, 887.
(d) Diao, T.; Pun, D.; Stahl, S. S. J. Am. Chem. Soc. 2013, 135, 8205.
(e) Bigi, M. A.; White, M. C. J. Am. Chem. Soc. 2013, 135, 7831.
[14] (a) Zhang, M.; Zhang, Y.; Jie, X.; Zhao, H.; Li, G.; Su, W. Org. Chem. Front. 2014, 1, 843.
(b) Wei, Y.; Hu, P.; Zhang, M.; Su, W. Chem. Rev. 2017.
[15] (a) Zhang, M.; Zhou, J.; Kan, J.; Wang, M.; Su, W.; Hong, M. Chem. Commun. 2010, 46, 5455.
(b) Zhou, J.; Wu, G.; Zhang, M.; Jie, X.; Su, W. Chem.-Eur. J. 2012, 18, 8032.
(c) Zhang, M.; Hu, P.; Zhou, J.; Wu, G.; Huang, S.; Su, W. Org. Lett. 2013, 15, 1718.
(d) Shang, Y.; Jie, X.; Zhou, J.; Hu, P.; Huang, S.; Su, W. Angew. Chem. Int. Ed. 2013, 52, 1299.
[16] Jie, X.; Shang, Y.; Zhang, X.; Su, W. J. Am. Chem. Soc. 2016, 138, 5623.
[17] Xiao, B.; Li, Y. M.; Liu, Z. J.; Yang, H. Y.; Fu, Y. Chem. Commun. 2012, 48, 4854.
[18] Klare, H. F.; Oestreich, M.; Ito, J.; Nishiyama, H.; Ohki, Y.; Tatsumi, K. J. Am. Chem. Soc. 2011, 133, 3312.
[19] Taylor, J. E.; Jones, M. D.; Williams, J. M. J.; Bull, S. D. Org. Lett. 2010, 12, 5740.
[20] Qi, T.; Qiu, W.; Liu, Y.; Zhang, H.; Gao, X.; Liu, Y.; Lu, K.; Du, C.; Yu, G.; Zhu, D. J. Org. Chem. 2008, 73, 4638.
/
| 〈 |
|
〉 |