

Chinese Journal of Organic Chemistry >
Iodine Catalyzed Kabachnik-Fields Reaction of Trialkyl Phosphites: Facile Access to Benzoxazine Containing Phosphorus
Received date: 2017-05-16
Revised date: 2017-07-13
Online published: 2017-08-16
Supported by
Project supported by the National Program on Key Basic Research Project (973 Program, No. 2012CBA01204), the National Natural Science Foundation of China (No. 21302084) and the Natural Science Foundation of Jiangxi Province (No. 20151BAB213007).
Iodine-catalyzed Kabachnik-Fields reaction with trialkyl phosphites for the synthesis of α-amino phosphates was developed. This transformation completes rapidly at 40℃, and is well tolerated with a range of amines and phosphites. Moreover, the products afforded by salicyl aldehydes with trialkyl phosphites could efficiently convert to benzoxazines containing phosphate under mild conditions, which provide a new precursor of new phenolic resin
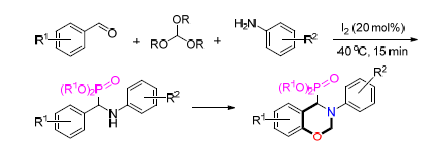
Key words: trialkyl phosphite; benzoxazineis; Kabachnik-Fields reaction; iodine
Wang Yufeng , Yang Yajie , Huang Ling , Jie Kun , Guo Shengmei , Cai Hua . Iodine Catalyzed Kabachnik-Fields Reaction of Trialkyl Phosphites: Facile Access to Benzoxazine Containing Phosphorus[J]. Chinese Journal of Organic Chemistry, 2017 , 37(12) : 3220 -3228 . DOI: 10.6023/cjoc201705023
[1] (a) Schmidt, R. R. Synthesis 1972, 7, 333.
(b) Burke, W. J. J. Am. Chem. Soc. 1949, 71, 609.
(c) Mueller, R.; Li, Y. X.; Hampson, A.; Zhong, S.; Harris, C.; Marrs, C.; Rachwal, R.; Ulas, J.; Nielsson, L.; Rogers, G. Bioorg. Med. Chem. Lett. 2011, 21, 3923.
(d) Dutta, A. K.; Gogoi, P.; Saikia, M.; Borah, P. R. Catal. Lett. 2016, 146, 902.
[2] (a) Bouaziz, Z.; Riondel, J.; Mey, A.; Berlion, M.; Villard, J.; Fillion, H. Eur. J. Med. Chem. 1991, 26, 469.
(b) Chylinska, J. B.; Urbanski, T.; Mordarski, M. J. Med. Chem. 1963, 6, 484.
(c) Benameur, L.; Bouaziz, Z.; Nebois, P.; Bartoli, M. H.; Boitard, M.; Fillion, H. Chem. Pharm. Bull. 1996, 44, 605.
(d) Mathew, B. P.; Kumar, A.; Sharma, S.; Shula, P. K.; Nath, M. Eur. J. Med. Chem. 2010, 45, 1502.
(e) Petrlkov, E.; Waisser, K.; DiviSova, H.; Husakov, P.; Vrabcova, P.; Kunes, J.; Kolr, K.; Stolarikov, J. Bioorg. Med. Chem. 2010, 18, 8178.
(f) Waghmode, N. A.; Kalbandhe, A. H.; Thorat, P. B.; Karade, N. N. Tetrahedron Lett. 2016, 57, 680.
[3] (a) Froimowicz, P.; Zhang, K.; Ishida, H. Chem.-Eur. J. 2016, 22, 2691.
(b) Liu, Y.-X.; Ma, H.-M.; Liu, Y.; Qiu, J.-J.; Liu, C.-M. Polymer 2016, 82, 32.
(c) Huang, C. C.; Lin, C. S.; Dai, S. A. RSC Adv. 2015, 5, 74874.
(d) Zhang, Q.; Yang, P.; Deng, Y.; Zhang, C.; Zhu, R.; Gu, Y. RSC Adv. 2015, 5, 103203.
(e) Gupta, K. S. V.; Ramana, D. V.; Vinayak, B.; Sridhar, B.; Chandrasekharam, M. New J. Chem. 2016, 40, 6389.
(f) Barta, P.; Szatmári, I.; Fülöp, F.; Heydenreich, M.; Koch, A.; Kleinpeter, E. Tetrahedron 2016, 72, 2402.
(g) Dumas, L.; Bonaud, L.; Olivier, M.; Poorteman, M.; Dubois, P. Eur. Polym. J. 2016, 75, 486.
(h) Wipt, P.; Hayes, G. B. Tetrahedron 1998, 54, 6987.
[4] (a) Su, H.; Liu, Z. J. Therm. Anal. Calorim 2013, 114, 1207.
(b) Lin, C. H.; Lin, H. T.; Sie, J. W.; Hwang, K. Y.; Tu, A. P. J. Polym. Sci.:Part A:Polym. Chem. 2010, 4555.
[5] (a) Kabachnik, M. I. Dokl. Akad. Nauk SSSR 1952, 83, 689.
(b) Fields, E. K. J. Am. Chem. Soc. 1952, 74, 1528
[6] (a) Wu, J.; Sun, W.; Wang, W.-Z.; Xia, H.-G. Chin. J. Chem. 2006, 24, 1054.
(b) Reddy, B. V. S.; Krishna, A. S.; Ganesh, A. V.; Kumar, J. J. S. N.Tetrahedron Lett. 2011, 52, 1369.
[7] Wu, J.; Sun, W.; Xia, H.-G.; Sun, X. Org. Biomol. Chem. 2006, 4, 1663.
[8] Jafari, A. A.; Nazarpour, M.; Abdollahi-Alibeik, M. Heteroat. Chem. 2010, 21, 397.
[9] Bhattacharya, T.; Majumdar, B.; Dey, D.; Sarma, T. K. RSC Adv. 2014, 4, 45831.
[10] Wu, J.; Sun, W.; Sun, X.; Xia, H.-G. Green Chem. 2006, 8, 365.
[11] (a) Ambica; Kumar, S.; Taneja, S. C.; Hundal, M. S.; Kapoor, K. K. Tetrahedron Lett. 2008, 49, 2208.
(b) Li, X.-C.; Gong, S.-S.; Zeng, D.-Y.; You, Y.-H.; Sun, Q. Tetrahedron Lett. 2016, 57, 1782.
(c) Manabe, K.; Kobayashi, S. Chem. Commun. 2000, 669.
(d) Qian, C.; Huang, T. J. Org. Chem. 1998, 63, 4125.
(e) Ranu, B. C.; Hajra, A.; Jana, U. Org. Lett. 1999, 1, 1141.
[12] (a) Tillu, V. H.; Dumbre, D. K.; Wakharkar, R. D.; Choudhary, V. R. Tetrahedron Lett. 2011, 52, 863.
(b) Kaboudin, B.; Nazari, R. Tetrahedron Lett. 2001, 42, 8211.
[13] (a) Mu, X.-J.; Lei, M.-Y.; Zou, J.-P.; Zhang, W. Tetrahedron Lett. 2006, 47, 1125.
(b) Bhattacharya, A. K.; Rana, K. C. Tetrahedron Lett. 2008, 49, 1782.
[14] (a) Ouahrouch, A.; Taourirte, M.; Schols, D.; Snoeck, R.; Andrei, G.; Angel, J. W.; Lazrek, H. B. Arch. Phram. Chem. Life Sci. 2016, 349, 30.
(b) Ouahrouch, A.; Krim, J.; Taourirte, M.; Lazrek, H. B.; Engels, J. W.; Bats, J. W. Acta Crystallogr. 2013, C69, 1157.
[15] (a) Thirumurugan, P.; Nandakumar, A.; SudhaPriya, N.; Muralidaran, D.; Perumal, P. T. Tetrahedron Lett. 2010, 51, 15708.
(b) Yadava, J. S.; Reddy, B. V. S.; Sreedhar, P. Green Chem. 2002, 4, 436.
(c) Disale, S. T.; Kale, S. R.; Kahandal, S. S.; Srinivasan, T. J.; Jayaram, R. V. Tetrahedron Lett. 2012, 53, 2277.
(d) Ordóñnez, M.; Sayago, F. J.; Cativiela, C. Tetrahedron 2012, 68, 6369.
[16] (a) Bhagat, S.; Chakraborti, A. K. J. Org. Chem. 2008, 73, 6029.
(b) Bhagat, S.; Chakraborti, A. K. J. Org. Chem. 2007, 72, 1263.
[17] Yu, Y.-Q.; Xu, D.-Z. Synthesis 2015, 47, 1869.
[18] Lee, S.; Park, J. H.; Kang, J.; Lee, J. K. Chem. Commun. 2001, 1698.
[19] Dar, B.; Singh, A.; Sahu, A.; Patida, P.; Chakraborty, A.; Sharma, M.; Singh, B. Tetrahedron Lett. 2012, 53, 5497.
[20] Kudrimoti, S.; Bommena, V. R. Tetrahedron Lett. 2005, 46, 1209
[21] (a) Huang, L.; Gong, J.; Zhu, Z.; Wang, Y.; Guo, S.; Cai, H. Org. Lett. 2017, 29, 2242.
(b) Huang, L.; Zhu, Z.; Cao, T.; Lei, X.; Gong, J.; Guo, S.; Cai, H. Chin. J. Org. Chem. 2017, 37, 1571(in Chinese).
(c) Gong, J.; Zhu, Z.; Lu, L.; Guo, S.; Cai, H. Chin. J. Org. Chem. 2015, 35, 1917(in Chinese).
(d) Gong, J.; Huang, L.; Deng, Q.; Jie, K.; Wang, Y.; Guo, S.; Cai, H. Org. Chem. Front. 2017, 4, DOI:10. 1039/C7QO00318H.
[22] Cambridge Crystallographic Data Centre (CCDC) for 4o (1509069) and 5q (1509068).
[23] (a) Sun, J.; Qiu, J.-K.; Jiang, B.; Hao, W.-J.; Guo, C.; Tu, S.-J. J. Org. Chem. 2016, 81, 3321.
(b) Ji, S.-J.; Wang, S.-Y.; Zhang Y.; Loh, T.-P. Tetrahedron 2004, 60, 2051.
(c) Zhang, H.; Wei, Q.; Zhu, G.; Qu, J.; Wang, B. Tetrahedron Lett. 2016, 57, 2633.
[24] Zhang, Y.;Zhu, C. Catal. Commun. 2011, 28, 134.
[25] Li, N.; Qiu, R.; Xu, X.; Chen, J.; Zhang, X.; Chen, S.; Yin, S. Catal. Commun. 2014, 43, 184.
[26] Thirmurugan, P.; Nandakumar, A.; Sudha, N.; Muralidaran, D.; Perumal, P. Tetrahedron. Lett. 2010, 51, 5708
[27] Song, L.; Yang, C.; Yu, Y.; Xu, D. Synthesis 2017, 49, 1641.
[28] Das, B.; Satyalakshmi, G.; Suneel, K.; Damodar, K. J. Org. Chem. 2009, 74, 8400.
[29] Shinde, p.; Kategaonkar, A.; Shingate, B.; Shingare, M. Tetrahedron Lett. 2011, 52, 2889. (Li, L.; Fan, Y.)
/
| 〈 |
|
〉 |