

Chinese Journal of Organic Chemistry >
Asymmetric Synthesis of Methyl N-(tert-Butoxycarbonyl)indoline-2-carboxylates
Received date: 2017-08-01
Revised date: 2017-08-31
Online published: 2017-09-08
Supported by
Project supported by the Natural Science Foundation of China (Nos. 81330075, 21172202).
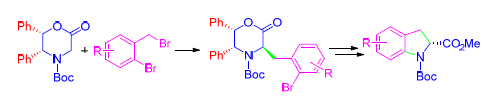
As a characteristic structural motif of numerous biologically active natural products and new drugs, chiral indoline derivatives have attracted much attention of chemists. Although many methods are available, there is still great need to develop a new, simple and highly efficient asymmetric synthetic method of indoline derivatives. Starting from Williams chiral auxiliary, a variety of methyl (R)-N-(tert-butoxycarbonyl)indoline-2-carboxylates were obtained with high overall yields and enantioselectivity through nucleophilic substitution, intramolecular Buchwald-Hartwig coupling reaction, etc.
Zhang Qianqian , Ding Qunshan , Song Chuanjun , Chang Junbiao . Asymmetric Synthesis of Methyl N-(tert-Butoxycarbonyl)indoline-2-carboxylates[J]. Chinese Journal of Organic Chemistry, 2018 , 38(1) : 221 -227 . DOI: 10.6023/cjoc201708002
[1] Bonjoch, J.; Solé, D.; García-Rubio, S.; Bosch, J. J. Am. Chem. Soc. 1997, 119, 7230.
[2] Zhang, D.; Song, H.; Qin, Y. Acc. Chem. Res. 2011, 44, 447.
[3] Deng, X.; Liang, K.; Tong, X.; Ding, M.; Zhou, A.; Xia, C. Org. Lett. 2014, 16, 3276.
[4] Wang, T.; Xu, Q.; Yu, P.; Liu, X.; Cook, J. Org. Lett. 2001, 3, 345.
[5] Fu, X.; Cook, J. M. J. Am. Chem. Soc. 1992, 114, 6910.
[6] Li, J.; Wang, T.; Yu, P.; Peterson, A.; Weber, R.; Soerens, D.; Grubisha, D.; Bennett, D.; Cook, J. M. J. Am. Chem. Soc. 1999, 121, 6998.
[7] Ori, M.; Toda, N.; Takami, K.; Tago, K.; Kogen, H. Tetrahedron 2005, 61, 2075.
[8] Toda, N.; Ori, M.; Takami, K.; Tago, K.; Kogen, H. Org. Lett. 2003, 5, 269.
[9] Langlois, N.; Gueritte, F.; Langlois, Y.; Potier, P. J. Am. Chem. Soc. 1976, 98, 7017.
[10] Kozmin, S. A.; Rawal, V. H. J. Am. Chem. Soc. 1998, 120, 13523.
[11] Marino, J. P.; Rubio, M. B.; Cao, G.; Dios, A. J. Am. Chem. Soc. 2002, 124, 13398.
[12] Iyengar, R.; Schildknegt, K.; Morton, M.; Aubé, J. J. Org. Chem. 2005, 70, 10645.
[13] Rakhit, A.; Hurley, M. E.; Tipnis, V.; Coleman, J.; Rommel, A.; Brunner, H. R. J. Clin. Pharm. 1986, 26, 156.
[14] Anas, S.; Kagan, H. B. Tetrahedron: Asymmetry 2009, 20, 2193.
[15] Zhang, L. B.; Qin, W.; Duan, Y. C.; Yu, B.; Zhang, E.; Liu, H. M. Chin. J. Org. Chem. 2012, 32, 1359(in Chinese).(张宝乐, 秦伟, 段迎超, 余斌, 张恩, 刘宏民, 有机化学, 2012, 32, 1359.)
[16] Sinclair, P. J.; Zhai, D.; Reibenspies, J.; Williams, R. M. J. Am. Chem. Soc. 1986, 108, 1103.
[17] Williams, R. M.; Sinclair, P. J.; Zhai, D.; Chen, D. J. Am. Chem. Soc. 1988, 110, 1547.
[18] Zhai, W. Tetrahedron 1988, 44, 5425.
[19] Zhai, D.; Zhai, W.; Williams, R. M. J. Am. Chem. Soc. 1988, 110, 2501.
[20] Williams, R. M.; Hendrix, J. A. J. Org. Chem. 1990, 55, 3723.
[21] Williams, R. M.; Im, M. N.; Cao, J. J. Am. Chem. Soc. 1991, 113, 6976.
[22] Williams, R. M.; Fegley, G. J. J. Org. Chem. 1993, 58, 6933.
[23] Song, C.; Tapaneeyakorn, S.; Murphy, A. C.; Butts, C.; Watts, A.; Willis, C. L. J. Org. Chem. 2009, 74, 8980.
[24] Williams, R. M. J. Org. Chem. 2011, 76, 4221.
[25] Yang, P. Y.; Zhou, Y. G. Tetrahedron: Asymmetry 2004, 15, 1145.
[26] Looper, R. E.; Williams, R. M. Tetrahedron Lett. 2001, 42, 769.
[27] Looper, R. E.; Runnegar, M. T. C.; Williams, R. M. Tetrahedron 2006, 62, 4549.
[28] Looper, R. E.; Williams, R. M. Angew. Chem., Int. Ed. 2004, 43, 2930.
[29] Looper, R. E.; Runnegar, M. T. C.; Williams, R. M. Angew. Chem., Int. Ed. 2005, 44, 3879.
[30] Onishi, T.; Sebahar, P. R.; Williams, R. M. Org. Lett. 2003, 5, 3135.
[31] Onishi, T.; Sebahar, P. R.; Williams, R. M. Tetrahedron 2004, 60, 9503.
[32] Ahrendt, K. A.; Williams, R. M. Org. Lett. 2004, 6, 4539.
[33] Jain, R. P.; Williams, R. M. Tetrahedron Lett. 2001, 42, 4437.
[34] Jain, R. P.; Williams, R. M. Tetrahedron 2001, 57, 6505.
[35] Schuber, P. T.; Williams, R. M. Tetrahedron Lett. 2012, 53, 380.
[36] Zhao, Y.; Liu, L.; Sun, W.; Lu, J.; McEachern, D.; Li, X.; Yu, S.; Bernard, D.; Ochsenbein, P.; Ferey, V.; Carry, J. C.; Dechamps, J. R.; Sun, D.; Wang, S. J. Am. Chem. Soc. 2013, 135, 7223.
[37] Kurokawa, M.; Sugai, T. Bull. Chem. Soc. Jpn. 2004, 77, 1021.
/
| 〈 |
|
〉 |