

Chinese Journal of Organic Chemistry >
Synthesis of Planar Chiral Ferrocenes via Transition-Metal-Catalyzed Direct C-H Bond Functionalization
Received date: 2017-08-15
Revised date: 2017-09-13
Online published: 2017-09-15
Supported by
Project supported by the National Key R&D Program of China (No. 2016YFA0202900), the National Basic Research Program of China (973 Program, No. 2015CB856600), the National Natural Science Foundation of China (Nos. 21332009, 21421091, 21572250), and the Chinese Academy Sciences (Nos. XDB20000000, QYZDY-SSW-SLH012).
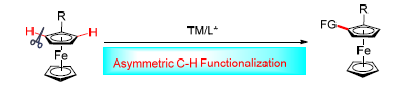
Ferrocenes bearing planar chirality have been demonstrated to be highly efficient ligands or catalysts in asymmetric catalysis. In view of their atom and step economies, direct asymmetric C—H bond functionalization is the most concise and powerful method for the construction of planar chiral ferrocenes compared with traditional approaches. This review summarizes recent progress on the development of novel methods to synthesize planar chiral compounds via transition- metal (Cu-, Pd-, Ir-, Rh-, Au-, Pt-) catalyzed asymmetric C—H bond functionalization. Preparation of a variety of new planar chiral ferrocene-based catalysts and ligands by utilizing these methods and their application in catalytic asymmetric reactions are also discussed.
Huang Jiapian , Gu Qing , You Shuli . Synthesis of Planar Chiral Ferrocenes via Transition-Metal-Catalyzed Direct C-H Bond Functionalization[J]. Chinese Journal of Organic Chemistry, 2018 , 38(1) : 51 -61 . DOI: 10.6023/cjoc201708030
[1] (a) Hayashi, T.; Togni, A. Ferrocenes, VCH, Weinheim, Germany, 1995.
(b) Togni, A.; Haltermann, R. L. Metallocenes, VCH, Weinheim, Germany, 1998.
(c) Štěpnicka, P. Ferrocenes, Wiley, Chichester, 2008.
(d) Dai, L.-X.; Hou, X.-L. Chiral Ferrocenes in Asymmetric Catalysis, Wiley, Weinheim, 2010.
[2] (a) Fu, G. C. Acc. Chem. Res. 2000, 33, 412.
(b) Wu, Y.-J.; Huo, S.-Q.; Gong, J.-F.; Cui, X.-L.; Ding, L.; Ding, K.-L.; Du, C.-X.; Liu, Y.-H.; Song, M.-P. J. Organomet. Chem. 2001, 637, 27.
(c) Dai, L.-X.; Tu, T.; You, S.-L.; Deng, W.-P.; Hou, X.-L. Acc. Chem. Res. 2003, 36, 659.
(d) Fu, G. C. Acc. Chem. Res. 2004, 37, 542.
(e) Atkinson, R. C. J.; Gibson, V. C.; Long, N. J. Chem. Soc. Rev. 2004, 33, 313.
(f) Hou, X.-L.; You, S.-L.; Tu, T.; Deng, W.-P.; Wu, X.-W.; Li, M.; Yuan, K.; Zhang, T.-Z.; Dai, L.-X. Top. Catal. 2005, 35, 87.
(g) Arrayás, R.-G.; Adrio, J.; Carretero, J.-C. Angew. Chem., Int. Ed. 2006, 45, 7674.
(h) Song, Q.-B.; Dong, Y. Chin. J. Org. Chem. 2007, 27, 66(in Chinese).(宋庆宝, 东宇, 有机化学, 2007, 27, 66.)
(i) Wu, Y.-J.; Yang, F.; Zhang, J.-L.; Cui, X.-L.; Gong, J.-F.; Song, M.-P.; Li, T.-S. Chin. Sci. Bull. 2010, 55, 2784.
(j) Noël, T.; Van der Eycken, J. Green Process. Synth. 2013, 2, 297.
(k) Schaarschmidt, D.; Lang, H. Organometallics 2013, 32, 5668.
[3] (a) Blaser, H.-U.; Brieden, W.; Pugin, B.; Spindler, F.; Studer, M.; Togni, A. Top. Catal. 2002, 19, 3.
(b) Blaser, H.-U.; Pugin, B.; Spindler, F. J. Mol. Catal. A 2005, 231, 1.
[4] (a) Battelle, L. F.; Bau, R.; Gokel, G. W.; Oyakawa, R. T.; Ugi, I. K. J. Am. Chem. Soc. 1973, 95, 482.
(b) Rebière, F.; Riant, O.; Ricard, L.; Kagan, H. B. Angew. Chem., Int. Ed. 1993, 32, 568.
(c) Richards, C. J.; Damalidis, T.; Hibbs, D. E.; Hursthouse, M. B. Synlett 1995, 74.
(d) Tsukazaki, M.; Tinkl, M.; Roglans, A.; Chapell, B. J.; Taylor, N. J.; Snieckus, V. J. Am. Chem. Soc. 1996, 118, 685.
(e) Enders, D.; Peters, R.; Lochtman, R.; Raabe, G. Angew. Chem., Int. Ed. 1999, 38, 2421.
(f) Laufer, R. S.; Veith, U.; Taylor, N. J.; Snieckus, V. Org. Lett. 2000, 2, 629.
(g) Bolm, C.; Kesselgruber, M.; Muniz, K.; Raabe, G. Organometallics 2000, 19, 1648.
(h) Bolm, C.; Kesselgruber, M.; Raabe, G. Organometallics 2002, 21, 707.
(i) Genet, C.; Canipa, S. J.; O'Brein, P.; Taylor, S. J. Am. Chem. Soc. 2006, 128, 9336.
For a review on kinetic resolution:(j) Alba, A.-N.; Rios, R. Molecules 2009, 14, 4747.
(k) Mercier, A.; Yeo, W. C.; Chou, J.; Chaudhuri, P. D.; Bernardinelli, G.; Kündig, E. P. Chem. Commun. 2009, 5227.
(l) Mercier, A.; Urbaneja, X.; Yeo, W. C.; Chaudhuri, P. D.; Cumming, G. R.; House, D.; Bernardinelli, G.; Kündig, E. P. Chem.-Eur. J. 2010, 16, 6285.
(m) Ogasawara, M.; Arae, S.; Watanabe, S.; Nakajima, K.; Takahashi, T. Chem.-Eur. J. 2013, 19, 4151.
[5] For reviews and book, see:(a) Giri, R.; Shi, B.-F.; Engle, K. M.; Maugel, N.; Yu, J.-Q. Chem. Soc. Rev. 2009, 38, 3242.
(b) Peng, H. M.; Dai, L.-X.; You, S.-L. Angew. Chem., Int. Ed. 2010, 49, 5826.
(c) Wencel-Delord, J.; Colobert, F. Chem.-Eur. J. 2013, 19, 14010.
(d) Engle, K. M.; Yu, J.-Q. J. Org. Chem. 2013, 78, 8927.
(e) Zheng, C.; You, S.-L. RSC Adv. 2014, 4, 6173.
(f) Ye, B.; Cramer, N. Acc. Chem. Res. 2015, 48, 1308.
(g) You, S.-L. Asymmetric Functionalization of C-H Bonds, RSC, Cambridge, U.K., 2015.
(h) Gao, D.-W.; Gu, Q.; Zheng, C.; You, S.-L. Acc. Chem. Res. 2017, 50, 351.
(i) Newton, C. G.; Wang, S.-G.; Oliveira, C. C.; Cramer, N. Chem. Rev. 2017, 117, 8908.
[6] (a) Wang, Y.; Zhang, A.; Liu, L.; Kang, J.; Zhang, F.; Ma, W. Chin. J. Org. Chem. 2015, 35, 1399(in Chinese).(王艳芳, 张安安, 刘澜涛, 康建勋, 张富强, 马文瑾, 有机化学, 2015, 35, 1399.)
(b) Arae, S.; Ogasawara, M. Tetrahedron Lett. 2015, 6, 1751.
(c) López, L. A.; López, E. Dalton. Trans. 2015, 44, 10128.
(d) Zhu, D.-Y.; Chen, P.; Xia, J.-B. ChemCatChem 2016, 8, 68.
[7] Xia, J.-B.; You, S.-L. Organometallics 2007, 26, 4869.
[8] Takebayashi, S.; Shizuno, T.; Otani, T.; Shibata, T. Beilstein J. Org. Chem. 2012, 8, 1844.
[9] Siegel, S.; Schmalz, H.-G. Angew. Chem., Int. Ed. 1997, 36, 2456.
[10] (a) Sokolov, V. I.; Troitskaya, L. L. Chimia 1978, 32, 122.
(b) Sokolov, V. I.; Troitskaya, L. L.; Reutov, O. A. J. Organomet. Chem. 1979, 182, 537.
(c) Günay, M. E.; Richards, C. J. Organometallics 2009, 28, 5833.
[11] (a) Shi, B.-F.; Maugel, N.; Zhang, Y.-H.; Yu, J.-Q. Angew. Chem., Int. Ed. 2008, 47, 4882.
(b) Shi, B.-F.; Zhang, Y.-H.; Lam, J. K.; Wang, D.-H.; Yu, J.-Q. J. Am. Chem. Soc. 2010, 132, 460.
(c) Wasa, M.; Engle, K. M.; Lin, D. W.; Yoo, E. J.; Yu, J.-Q. J. Am. Chem. Soc. 2011, 133, 19598.
(d) Musaev, D. G.; Kaledin, A.; Shi, B.-F.; Yu, J.-Q. J. Am. Chem. Soc. 2012, 134, 1690.
(e) Xiao, K.-J.; Lin, D. W.; Miura, M.; Zhu, R.-Y.; Gong, W.; Wasa, M.; Yu, J.-Q. J. Am. Chem. Soc. 2014, 136, 8138.
(f) Chu, L.; Xiao, K.-J.; Yu, J.-Q. Science 2014, 346, 451.
(g) Chan, K. S. L.; Fu, H.-Y.; Yu, J.-Q. J. Am. Chem. Soc. 2015, 137, 2042.
(h) Laforteza, B. N.; Chan, K. S. L.; Yu, J.-Q. Angew. Chem., Int. Ed. 2015, 54, 11143.
(i) Xiao, K.-J.; Chu, L.; Yu, J.-Q. Angew. Chem., Int. Ed. 2016, 55, 2856.
(j) Xiao, K.-J.; Chu, L.; Chen, G.; Yu, J.-Q. J. Am. Chem. Soc. 2016, 138, 7796.
[12] Gao, D.-W.; Shi, Y.-C.; Gu, Q.; Zhao, Z.-L.; You, S.-L. J. Am. Chem. Soc. 2013, 135, 86.
[13] Cheng, G.-J.; Chen, P.; Sun, T.-Y.; Zhang, X.; Yu, J.-Q.; Wu, Y.-D. Chem. Eur. J. 2015, 21, 11180.
[14] Gair, J.-J.; Haines, B.-E.; Filatov, A.-S.; Musaev, D.-G.; Lewis, J.-C. Chem. Sci. 2017, 8, 5746.
[15] Pi, C.; Li, Y.; Cui, X.-L.; Zhang, H.; Han, Y.-B.; Wu, Y.-J. Chem. Sci. 2013, 4, 2675.
[16] Shi, Y.-C.; Yang, R.-F.; Gao, D.-W.; You, S.-L. Beilstein J. Org. Chem. 2013, 9, 1891.
[17] Zhang, H.; Cui, X.-L.; Yao, X.-Y.; Wang, H.; Zhang, J.-Y.; Wu, Y.-J. Org. Lett. 2012, 14, 3012.
[18] Gao, D.-W.; Gu, Q.; You, S.-L. J. Am. Chem. Soc. 2016, 138, 2544.
[19] Pi, C.; Cui, X.-L.; Liu, X.-Y.; Guo, M.X.; Zhang, H.-Y.; Wu, Y.-J. Org. Lett. 2014, 16, 5164.
[20] Gao, D.-W.; Yin, Q.; Gu, Q.; You, S.-L. J. Am. Chem. Soc. 2014, 136, 4841.
[21] Fukuzawa, S.-I.; Yamamoto, M.; Hosaka, M.; Kikuchi, S. Eur. J. Org. Chem. 2007, 5540.
[22] Deng, R.; Huang, Y.; Ma, X.; Li, G.; Zhu, R.; Wang, B.; Kang, Y.-B.; Gu, Z. J. Am. Chem. Soc. 2014, 136, 4472.
[23] Zhang, S.; Lu, J.; Ye, J.; Duan, W.-L. Chin. J. Org. Chem. 2016, 36, 752(in Chinese).(张松, 陆俊筑, 叶金星, 段伟良, 有机化学, 2016, 36, 752.)
[24] (a) Nottingham, C.; Müller-Bunz, H.; Guiry, P. J. Angew. Chem., Int. Ed. 2016, 55, 11115.
(b) Nottingham, C.; Müller-Bunz, H.; McGlinchey, M. J.; Guiry, P. J. Eur. J. Org. Chem. 2017, 2848.
[25] (a) Ma, X.; Gu, Z. RSC. Adv. 2014, 4, 36241.
(b) Liu, L.; Zhang, A.-A.; Zhao, R.-J.; Li, F.; Meng, T.-J.; Ishida, N.; Murakami, M.; Zhao, W.-X. Org. Lett. 2014, 16, 5336.
[26] Gao, D.-W.; Zheng, C.; Gu, Q.; You, S.-L. Organometallics 2015, 34, 4618.
[27] Gao, D.-W.; Gu, Y.; Wang, S.-B.; Gu, Q.; You, S.-L. Organometallics 2016, 35, 3227.
[28] (a) Shibata, T.; Shizuno, T. Angew. Chem., Int. Ed. 2014, 53, 5410.
(b) Takebayashi, S.; Shibata, T. Organometallics 2012, 31, 4114.
[29] (a) Shibata, T.; Shizuno, T.; Sasaki, T. Chem. Commun. 2015, 51, 7802.
(b) Zhang, Q.-W.; An, K.; Liu, L.-C.; Yue, Y.; He, W. Angew. Chem., Int. Ed. 2015, 54, 6918.
(c) Murai, M.; Matsumoto, K.; Takeuchi, Y.; Takai, K. Org. Lett. 2015, 17, 3102.
[30] Wang, S.-B.; Zheng, J.; You, S.-L. Organometallics 2016, 35, 1420.
[31] Guimond, N.; Gouliaras, C.; Fagnou, K. J. Am. Chem. Soc. 2010, 132, 6908.
[32] Urbano, A.; Hernández-Torres, G.; del Hoyo, A. M.; Martínez-Carrión, A.; Carreño, M. C. Chem. Commun. 2016, 52, 6419.
[33] Shibata, T.; Uno, N.; Sasaki, T.; Kanyiva, K. S. J. Org. Chem. 2016, 81, 6266.
[34] Fürstner, A.; Mamane, V. J. Org. Chem. 2002, 67, 6264.
/
| 〈 |
|
〉 |