

Chinese Journal of Organic Chemistry >
Synthesis and Antimicrobial Activities of Novel 1,2,4-Triazole-acyl-hydrazone Derivatives Containing the Quinazolin-4-one Moiety
Received date: 2017-08-24
Revised date: 2017-09-28
Online published: 2017-10-20
Supported by
Project supported by the National Natural Science Foundation of China (No. 21362003) and the Agricultural Research Projects of Guizhou Province (No. 20093010).
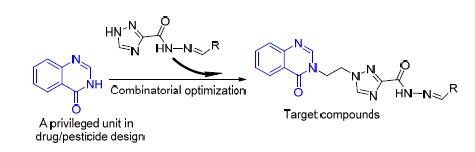
A total of twenty novel 1, 2, 4-triazole-acylhydrazone derivatives containing the quinazolin-4-one moiety were synthesized via the condensation reaction of triazole hydrazide with various aromatic aldehydes, and fully characterized by 1H NMR, 13C NMR and HRMS spectra. Antimicrobial assays in vitro indicated that most of the target compounds exhibited good antibacterial activities against the pathogenic phytobacteria Xanthomonas oryzae pv. oryzae(Xoo) and Xanthomonas axonopodis pv. citri(Xac). Notably, N'-(2-methoxybenzylidene)-1-(2-(4-oxoquinazolin-3(4H)-yl) ethyl)-1H-1, 2, 4-triazole-3-acylhy-drazone)(7q) displayed the inhibition rate of 100% against the above two bacteria at 100 μg/mL. Additionally, 1-(2-(4-oxo-quinazolin-3(4H)-yl) ethyl)-N'-(4-(trifluoromethyl) benzylidene)-1H-1, 2, 4-triazole-3-acylhydrazone (7i), N'-(2-bromobenzyli-dene)-1-(2-(4-oxoquinazolin-3(4H)-yl) ethyl)-1H-1, 2, 4-triazole-3-acylhydrazone (7j), N'-(4-bromobenzylidene)-1-(2-(4-oxo-quinazolin-3(4H)-yl) ethyl)-1H-1, 2, 4-triazole-3-acylhydrazone (7l), and N'-(4-methoxybenzylidene)-1-(2-(4-oxoquinazolin-3(4H)-yl) ethyl)-1H-1, 2, 4-triazole-3-acylhydrazone (7o) were found to possess the inhibition rate of >55% against the fungus Botrytis cinerea Pers. at 50 μg/mL.
Du Huan , Fan Zhijiang , Yang Lan , Bao Xiaoping . Synthesis and Antimicrobial Activities of Novel 1,2,4-Triazole-acyl-hydrazone Derivatives Containing the Quinazolin-4-one Moiety[J]. Chinese Journal of Organic Chemistry, 2018 , 38(2) : 531 -538 . DOI: 10.6023/cjoc201708051
[1] Sun, X.; Cao, Y.; Yang, Z.; Xu, C.; Li, X.; Wang, S.; Zhang, Q. Plant J. 2004, 37, 517.
[2] Graham, J. H.; Gottwald, T. R.; Cubero, J.; Achor, D. S. Mol. Plant Pathol. 2004, 5, 1.
[3] Salanoubat M.; Genin S.; Artiguenave F.; Gouzy J.; Mangenot S.; Arlat M.; Billaultk A.; Brottier P.; Camus J. C.; Cattolico L.; Chandler M.; Choisne N.; Claudel-Renard C.; Cunnac S.; Demange, N.; Gaspin C.; Lavie M.; Moisan A.; Robert C.; Saurin W.; Schiex T.; Siguier P.; Thébault P.; Whalen M.; Wincker P.; Levy M.; Weissenbach J.; Boucher C. A. Nature 2002, 415, 497.
[4] Ryan R. P.; Vorhölter F. J.; Potnis N.; Jones J. B.; Van Sluys M. A.; Bogdanove A. J.; Dow J. M. Nat. Rev. Microbiol. 2011, 9, 344.
[5] Li J.; Wang N. PLoS One 2011, 6, e21804.
[6] Chang, Y. H.; Zhang, L.; Liu Y. Z.; Chen, Z. Y. Jiangsu Agric. Sci. 2012, 40, 89(in Chinese). (常有宏, 张磊, 刘邮洲, 陈志谊, 江苏农业科学, 2012, 40, 89.)
[7] Sun, N.; Du, R. L.; Zheng, Y. Y.; Huang, B. H.; Guo, Q.; Zhang, R. F.; Wong, K. Y.; Lu, Y. J. Eur. J. Med. Chem. 2017, 135, 1.
[8] Shavit, M.; Pokrovskaya, V.; Belakhov, V.; Baasov, T. Bioorg. Med. Chem. 2017, 25, 2917.
[9] Zhang, F.; Wen, Q.; Wang, S. F.; Shahla, K. B.; Yang, Y. S.; Liu, J. J.; Zhang, W. M.; Zhu, H. L. Bioorg. Med. Chem. Lett. 2014, 24, 90.
[10] Somagond, S. M.; Kamble, R. R.; Kattimani, P. P.; Joshi, S. D.; Dixit, S. R. Heterocycl. Commun. 2017, 23, 317.
[11] Kulabas, N.; Tatar, E.; Özakpinar, Ö. B.; Özsavci, D.; Pannecouque, C.; Clercq, E. D.; Küçükgüzel, I. Eur. J. Med. Chem. 2016, 121, 58.
[12] Basaran, E.; Karakucuk-Iyidogan, A.; Schols, D.; Oruc-Emre, E. E. Chirality 2016, 28, 495.
[13] Xiong, Q.; Liu, J.; Lin, X.; Bao, X. Chin. J. Org. Chem. 2012, 32, 1951(in Chinese). (熊启中, 刘军虎, 林选福, 鲍小平, 有机化学, 2012, 32, 1951.)
[14] Che, Z.; Zhang, S.; Shao, Y.; Fan, L.; Xu, H.; Yu, X.; Zhi, X. Y.; Yao, X. J.; Zhang, R. J. Agric. Food Chem. 2013, 61, 5696.
[15] Wang, X.; Yin, J.; Shi, L.; Zhang, G. P.; Song, B. A. Eur. J. Med. Chem. 2014, 77, 65.
[16] Zhang, J.; Liu, J.; Ma, Y.; Ren, D.; Cheng, P.; Zhao, J. W.; Zhang, F.; Yao, Y. Bioorg. Med. Chem. Lett. 2016, 26, 2273.
[17] Ding, P. P.; Gao, M.; Mao, B. B.; Cao, S. L.; Liu, C. H.; Yang, C. R.; Li, Z. F.; Liao, J.; Zhao, H.; Li, Z.; Li, J.; Wang, H.; Xu, X. Eur. J. Med. Chem. 2016, 108, 364.
[18] Chen, M.; Li, P.; Hu, D.; Zeng, S.; Li, T.; Jin, L.; Xue, W.; Song, B. Bioorg. Med. Chem. Lett. 2016, 26, 168.
[19] Zhu, S. S.; Lu, J. R.; Xin, C. W.; Lu, B. W.; Bao, X. R.; Zou, M.; Liu, Q. Chem. J. Chin. Univ. 2010, 31, 2228(in Chinese). (朱姗姗, 卢俊瑞, 辛春伟, 卢博为, 鲍秀荣, 邹敏, 刘倩, 高等学校化学学报, 2010, 31, 2228.)
[20] Zhang, M.; Lu, J.; Xin, C.; Liu, F.; Wang, J.; Li, H.; Wei, R.; Bao, X. Chin. J. Org. Chem. 2009, 29, 1645(in Chinese). (张明, 卢俊瑞, 辛春伟, 刘芳, 王菁菁, 李红姬, 魏荣宝, 鲍秀荣, 有机化学, 2009, 29, 1645.)
[21] Liu, J.; Liu, Y.; Jian, J.; Bao, X. Chin. J. Org. Chem. 2013, 33, 370(in Chinese). (刘军虎, 刘勇, 蹇军友, 鲍小平, 有机化学, 2013, 33, 370.)
[22] Yan, B.; Lv, X.; Du, H.; Bao, X. Chin. J. Org. Chem. 2016, 36, 207(in Chinese). (闫柏任, 吕新阳, 杜欢, 鲍小平, 有机化学, 2016, 36, 207.)
[23] Yan, B. R.; Lv, X. Y.; Du, H.; Gao, M. N.; Huang, J.; Bao, X. P. Chem. Pap. 2016, 70, 983.
[24] Zhang, G.; Fu, X.; Peng, X.; Li, X.; Chen, J. J. Chem. Res. 2013, 37, 730.
[25] Yao, Y. P.; Dai, F. Y.; Dong, K. K.; Mao, Q.; Wang, Y. L.; Chen, T. J. Chem. Res. 2011, 35, 4.
[26] Okuda, K.; Ohtomo, H.; Tagata, T.; Hirota, T.; Sasaki, K. Synth. Commun. 2011, 41, 812.
[27] Yang, L.; Bao, X. RSC Adv. 2017, 7, 34005.
[28] Fan, Z.; Shi, Z.; Zhang, H.; Liu, X.; Bao, L.; Ma, L.; Zuo, X.; Zheng, Q.; Mi, N. J. Agric. Food Chem. 2009, 57, 4279.
[29] Chen, C. J.; Song, B. A.; Yang, S.; Xu, G. F.; Bhadury, P. S.; Jin, L. H.; Hu, D. Y.; Li, Q. Z.; Liu, F.; Xue, W.; Lu, P.; Chen, Z. Bioorg. Med. Chem. 2007, 15, 3981.
/
| 〈 |
|
〉 |