

Chinese Journal of Organic Chemistry >
Progress in the Synthesis of Arylated Coumarin Derivatives
Received date: 2017-08-29
Revised date: 2017-09-30
Online published: 2017-10-20
Supported by
Project supported by the National Natural Science Foundation of China (No. 21302042), the Natural Science and Technology Foundation of Department of Henan Province (No. 172102210225), and the Natural Science Foundation in Henan Province Department of Education (No. 17A150005).
Arylated coumarin derivatives are a kind of heterocyclic compounds, which play important roles in medicine, biology and material science, and their synthetic methods have attracted much attention. In recent years, many efficient, green synthetic approaches of arylated coumarin derivatives have been reported using transition-metal or metal-free catalytic systems. The recent progress in the synthesis of arylated coumarin derivatives is reviewed according to differences of reaction positions and arylation sources.
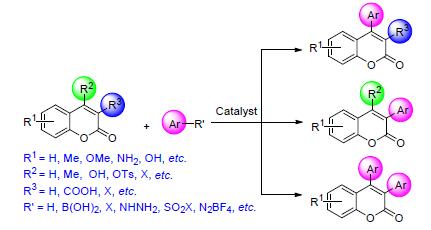
Key words: coumarin; transition-metal catalyst; arylation; synthesis; research progress
Liu Shuainan , Yuan Jinwei , Qu Lingbo . Progress in the Synthesis of Arylated Coumarin Derivatives[J]. Chinese Journal of Organic Chemistry, 2018 , 38(2) : 316 -327 . DOI: 10.6023/cjoc201708058
[1] Sekino, E.; Kumamoto, T.; Tanaka, T.; Ikeda, T.; Ishikawa, T. J. Org. Chem. 2004, 69, 2760.
[2] Anand, P.; Singh, B.; Singh, N. Bioorg. Med. Chem. 2012, 20, 1175.
[3] Musa, M. A.; Cooperwood, S.; Khan, M.; Omar, F. Curr. Med. Chem. 2008, 15, 2664.
[4] Ma, Y.; Luo, W.; Quinn, P. J. J. Med. Chem. 2004, 47, 6349.
[5] Yuan, J. W.; Li, Y. Z.; Yang, L. R.; Mai, W. P.; Mao, P.; Xiao, Y. M.; Qu, L. B. Tetrahedron 2015, 71, 8178.
[6] Mi, X.; Huang, M. M.; Zhang, J. Y.; Wang, C. Y.; Wu, Y. J. Org. Lett. 2013, 15, 6266.
[7] Mashelkar, U. C.; Audi, A. A. J. Indian Chem. Soc. 2005, 82, 258.
[8] Ganguly, N.; Sukai, A. K.; De, S. Synth. Commun. 2001, 31, 301.
[9] Gavara, L.; Boisse, T.; Rigo, B.; Hénichart, J. P. Tetrahedron 2008, 64, 4999.
[10] Thapliyal, P. C.; Singh, P. K.; Khanna, R. N. Synth. Commun. 1993, 23, 2821.
[11] Wang, H.; Zhou, S. L.; Guo, L. N.; Duan, X. H. Tetrahedron 2015, 71, 630.
[12] Yuan, J. W.; Yin, Q. Y.; Yang, L. R.; Mai, W. P.; Mao, P.; Xiao, Y. M.; Qu, L. B. RSC Adv. 2015, 5, 88258.
[13] Adib, M.; Rajai-Daryasarei, S.; Pashazadeh, R.; Tajik, M.; Mirzaei, P. Tetrahedron Lett. 2016, 57, 3701.
[14] Jafarpour, F.; Abbasnia, M. J. Org. Chem. 2016, 81, 11982.
[15] Cremlyn, R. J.; Clowes, S. M. J. Chem. Soc. Pak. 1988, 10, 97.
[16] Liu, M.; Liu, Y.; Liu, A. L.; Zhang, D. K.; Chen, M. G.; Wu, C. C.; Hua, X. W.; Zhou, S.; Li, Z. M. Chin. J. Org. Chem. 2016, 36, 1653(in Chinese). (刘明, 刘阳, 刘艾林, 张冬凯, 陈明桂, 吴长春, 华学文, 周莎, 李正名, 有机化学, 2016, 36, 1653.)
[17] Dian, L. Y.; Zhao, H.; Zhang-Negrerie, D.; Du, Y. F. Adv. Synth. Catal. 2016, 358, 2422.
[18] Niu, B.; Zhao, W.; Ding, Y.; Bian, Z.; Pittman Jr, C. U.; Zhou, A.; Ge, H. J. Org. Chem. 2015, 80, 7251.
[19] Yuan, J. W.; Yang, L. R.; Yin, Q. Y.; Mao, P.; Qu, L. B. RSC Adv. 2016, 6, 35936.
[20] Wang, C.; Wu, C.; Zhu, J.; Miller, R. H.; Wang, Y. J. Med. Chem. 2011, 54, 2331.
[21] Wang, S. J.; Li, C. W.; Li, J.; Chen, B.; Guo, Y. Acta Chim. Sinica 2017, 75, 383(in Chinese). (王少静, 李长伟, 李锦, 陈邦, 郭媛, 化学学报, 2017, 75, 383.)
[22] Li, C. W.; Yang, D.; Yin, B.; Guo, Y. Chin. J. Org. Chem. 2016, 36, 787(in Chinese). (李长伟, 杨栋, 尹兵, 郭媛, 有机化学, 2016, 36, 787.)
[23] Schiedel, M. S.; Briehn, C. A.; Bäuerle, P. Angew. Chem., Int. Ed. 2001, 40, 4677.
[24] Matos, M. J.; Vazquez-Rodriguez, S.; Borges, F.; Santana, L.; Uriarte, E. Tetrahedron Lett. 2011, 52, 1225.
[25] Pérez-Cruz, F.; Serra, S.; Delogu, G.; Lapier, M.; Maya, J. D.; Olea-Azar, C.; Santana, L.; Uriarte, E. Bioorg. Med. Chem. Lett. 2012, 22, 5569.
[26] Serra, S.; Chicca, A.; Delogu, G.; Vázquez-Rodríguez, S.; Santana, L.; Uriarte, E.; Casu, L.; Gertsch, J. Bioorg. Med. Chem. Lett. 2012, 22, 5791.
[27] Delogu, G. L.; Serra, S.; Quezada, E.; Uriarte, E.; Vilar, S.; Tatonetti, N. P.; Viña, D. ChemMedChem 2014, 9, 1672.
[28] Zhu, Q.; Wu, J.; Fathi, R.; Yang, Z. Org. Lett. 2002, 4, 3333.
[29] Zhao, H. P.; Yan, B.; Peterson, L. B.; Blagg, B. S. J. ACS Med. Chem. Lett. 2012, 3, 327.
[30] Meimetis, L. G.; Carlson, J. C. T.; Giedt, R. J.; Kohler, R. H.; Weissleder, R. Angew. Chem., Int. Ed. 2014, 53, 7531.
[31] Paul, S.; Pradhan, K.; Ghosh, S.; De, S. K.; Das, A. R. Adv. Synth. Catal. 2014, 356, 1301.
[32] Rodríguez, S. A.; Baumgartner, M. T. Tetrahedron Lett. 2010, 51, 5322.
[33] Martins, S.; Branco, P. S.; de la Torre, M. C.; Sierra, M. A.; Pereira, A. Synlett 2010, 2918.
[34] Messaoudi, S.; Brion, J. D.; Alami, M. Org. Lett. 2012, 14, 1496.
[35] Carrër, A.; Brion, J. D.; Messaoudi, S.; Alami, M. Adv. Synth. Catal. 2013, 355, 2044.
[36] Jafarpour, F.; Zarei, S.; Barzegar, M.; Olia, A.; Jalalimanesh, N.; Rahiminejadan, S. J. Org. Chem. 2013, 78, 2957.
[37] Unsinn, A.; Wunderlich, S. H.; Knochel, P. Adv. Synth. Catal. 2013, 355, 989.
[38] Wunderlich, S. H.; Knochel, P. Angew. Chem., Int. Ed. 2007, 46, 7685.
[39] Yakushiji, F.; Haramo, M.; Miyadera, Y.; Uchiyama, C.; Takayama, K.; Hayashi, Y. Tetrahedron Lett. 2014, 55, 3316.
[40] Pardo, L. M.; Prendergast, A. M.; Nolan, M. T.; Muimhneacháin, E. Ó.; McGlacken, G. P. Eur. J. Org. Chem. 2015, 2015, 3540.
[41] Chauhan, P.; Ravi, M.; Singh, S.; Prajapati, P.; Yadav, P. P. RSC Adv. 2016, 6, 109.
[42] Yuan, J. W.; Li, W. J.; Yang, L. R.; Mao, P.; Xiao, Y. M. Z. Naturforsch. B 2016, 71, 1115.
[43] Jafarpour, F.; Barzegar, M.; Olia, A.; Hazrati, H. Adv. Synth. Catal. 2013, 355, 3407.
[44] Kojima, M.; Oisaki, K.; Kanai, M. Chem. Commun. 2015, 51, 9718.
[45] Tucker, J. W.; Stephenson, C. R. J. J. Org. Chem. 2012, 77, 1617.
[46] Teegardin, K.; Day, J. I.; Chan, J.; Weaver, J. Org. Process Res. Dev. 2016, 20, 1156.
[47] Skubi, K. L.; Blum, T. R.; Yoon, T. P. Chem. Rev. 2016, 116, 10035.
[48] Hari, D. P.; König, B. Org. Lett. 2011, 13, 3852.
[49] Schroll, P.; Hari, D. P.; König, B. ChemistryOpen 2012, 1, 130.
[50] Schroll, P.; Fehl, C.; Dankesreiter, S.; König, B. Org. Biomol. Chem. 2013, 11, 6510.
[51] Rybicka-Jasińska, K.; König, B.; Gryko, D. Eur. J. Org. Chem. 2017, 2104.
[52] Meng, J. B.; Shen, M. G.; Fu, D. C.; Gao, Z. H.; Wang, R. J.; Wang, H. G.; Matsuura, T. Synthesis 1990, 719.
[53] Jafarpour, F.; Hazrati, H.; Mohasselyazdi, N.; Khoobi, M.; Shafiee, A. Chem. Commun. 2013, 49, 10935.
[54] She, Z.; Shi, Y.; Huang, Y.; Cheng, Y.; Song, F.; You, J. Chem. Commun. 2014, 50, 13914.
[55] Kozyrod, R. P.; Pinhey, J. T. Aust. J. Chem. 1985, 38, 1155.
[56] Barton, D. H. R.; Donnelly, D. M. X.; Finet, J. P.; Guiry, P. J. Tetrahedron Lett. 1989, 30, 1539.
[57] Barton, D. H. R.; Donnelly, D. M. X.; Finet, J. P.; Stenson, P. H. Tetrahedron 1988, 44, 6387.
[58] Barton, D. H. R.; Donnelly, D. M. X.; Finet, J. P.; Guiry, P. J. J. Chem. Soc., Perkin Trans. 1 1992, 1365.
[59] Najib, A.; Tabuchi, S.; Hirano, K.; Miura, M. Heterocycles 2016, 92, 1187.
[60] Tang, Z. Y.; Hu, Q. S. Adv. Synth. Catal. 2004, 346, 1635.
[61] Luo, Y.; Wu, J. Tetrahedron Lett. 2009, 50, 2103.
[62] Kuroda, J. I.; Inamoto, K.; Hiroya, K.; Doi, T. Eur. J. Org. Chem. 2009, 2251.
[63] Xing, C. H.; Lee, J. R.; Tang, Z. Y.; Zheng, J. R.; Hu, Q. S. Adv. Synth. Catal. 2011, 353, 2051.
[64] Wong, P. Y.; Chow, W. K.; Chung, K. H.; So, C. M.; Lau, C. P.; Kwong, F. Y. Chem. Commun. 2011, 47, 8328.
[65] Santana, M. D.; García-Bueno, R.; García, G.; Sánchez, G.; García, J.; Kapdi, A. R.; Naik, M.; Pednekar, S.; Pérez, J.; García, L.; Pérez, E.; Serrano, J. L. Dalton Trans. 2012, 41, 3832.
[66] Li, Y. M.; Qi, Z. S.; Wang, H. F.; Fu, X. M.; Duan, C. Y. J. Org. Chem. 2012, 77, 2053.
[67] Khoobi, M.; Alipour, M.; Zarei, S.; Jafarpour, F.; Shafiee, A. Chem. Commun. 2012, 48, 2985.
[68] Shah, P.; Santana, M. D.; García, J.; Serrano, J. L.; Naik, M.; Pednekar, S.; Kapdi, A. R. Tetrahedron 2013, 69, 1446.
[69] Khoobi, M.; Molaverdi, F.; Alipour, M.; Jafarpour, F.; Foroumadi, A.; Shafiee, A. Tetrahedron 2013, 69, 11164.
[70] Cheval, N. P.; Dikova, A.; Blanc, A.; Weibel, J. M.; Pale, P. Chem. -Eur. J. 2013, 19, 8765.
[71] Dikova, A.; Cheval, N. P.; Blanc, A.; Weibel, J. M.; Pale, P. Adv. Synth. Catal. 2015, 357, 4093.
[72] Mutai, P.; Breuzard, G.; Pagano, A.; Allegro, D.; Peyrot, V.; Chibale, K. Bioorg. Med. Chem. 2017, 25, 1652.
[73] Min, M.; Hong, S. Chem. Commun. 2012, 48, 9613.
[74] Wu, J.; Yang, Z. J. Org. Chem. 2001, 66, 7875.
[75] Rao, M. L. N.; Venkatesh, V.; Jadhav, D. N. Eur. J. Org. Chem. 2010, 3945.
[76] Rao, M. L. N.; Kumar, A. Tetrahedron 2014, 70, 6995.
[77] Gao, W.; Luo, Y.; Ding, Q.; Peng, Y.; Wu, J. Tetrahedron Lett. 2010, 51, 136.
[78] Rieke, R. D.; Kim, S. H. Tetrahedron Lett. 2011, 52, 3094.
[79] Wu, J.; Zhang, L.; Xia, H. G. Tetrahedron Lett. 2006, 47, 1525.
[80] Wu, J.; Zhang, L.; Luo, Y. Tetrahedron Lett. 2006, 47, 6747.
[81] Zhang, L.; Meng, T.; Fan, R.; Wu, J. J. Org. Chem. 2007, 72, 7279.
[82] Rao, M. L. N.; Kumar, A. Tetrahedron 2015, 71, 5137.
/
| 〈 |
|
〉 |