

Chinese Journal of Organic Chemistry >
Ag-Catalyzed Monofluoromethylation of Purin-9-yl Allenes with Fluorobis(phenylsulfonyl)methane
Received date: 2017-11-01
Revised date: 2017-11-19
Online published: 2017-11-21
Supported by
Project supported by the National Natural Science Foundation of China (Nos. U1604283, 21402041), the Plan for Scientific Innovation Talent of Henan Province (No. 164200510008), the China Postdoctoral Science Foundation Funded Project (No. 2016M592293), the Program for Innovative Research Team in Science, Technology in University of Henan Province (No. 15IRTSTHN003) and the Program of Introducing Talents of Discipline to Universities (111 Project, No. D17007).
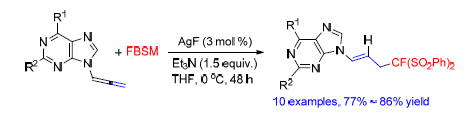
The monofluoromethylation of purin-9-yl allenes with fluorobis(phenylsulfonyl)methane has been achieved. With AgF (3 mol%) as the catalyst, the fluorobis(phenylsulfonyl)methylated adducts could be afforded in excellent yields. The monofluoromethylation exhibited high chemoselectivities and E-selectivies. Meanwhile, the monofluoromethylation of purin-9-yl allenes with fluorobis(phenylsulfonyl)methane provided a useful route to construct fluorinated acyclic nucleoside analogues.
Guo Zhen , Xie Mingsheng , Han Ruijie , Qu Guirong , Guo Haiming . Ag-Catalyzed Monofluoromethylation of Purin-9-yl Allenes with Fluorobis(phenylsulfonyl)methane[J]. Chinese Journal of Organic Chemistry, 2018 , 38(1) : 112 -117 . DOI: 10.6023/cjoc201711001
[1] (a) Smart, B. E. J. Fluorine Chem. 2001, 109, 3.
(b) Kirsch, P. Modern Fluoroorganic Chemistry, Wiley-VCH, Weinheim, Germany, 2004.
(c) Kirk, K. L. J. Fluorine Chem. 2006, 127, 1013.
(d) Li, Y.; Ni, C.; Liu, J.; Zhang, L.; Zheng, J.; Zhu, L.; Hu, J. Org. Lett. 2006, 8, 1693.
(e) Bégué, J.-P.; Bonnet-Delpon, D. J. Fluorine Chem. 2006, 127, 992.
(f) Prakash, G. K. S.; Hu, J. Acc. Chem. Res. 2007, 40, 921.
(g) Müller, K.; Faeh, C.; Diederich, F. Science 2007, 317, 1881.
(h) Purser, S.; Moore, P. R.; Swallow, S.; Gouverneur, V. Chem. Soc. Rev. 2008, 37, 320.
(i) Hu, J.; Zhang, W.; Wang, F. Chem. Commun. 2009, 7465.
(j) Liao, F.-M.; Yu, J.-S.; Zhou, J. Chin. J. Org. Chem. 2017, 37, 2175(in Chinese).(廖富民, 余金生, 周剑, 有机化学, 2017, 37, 2175.)
(k) Liao, F.-M.; Cao, Z.-Y.; Yu, J.-S.; Zhou, J. Angew. Chem., Int. Ed. 2017, 56, 2459.
[2] Fukuzumi, T.; Shibata, N.; Sugiura, M.; Yasui, H.; Nakamura, S.; Toru, T. Angew. Chem., Int. Ed. 2006, 45, 4973
[3] Ni, C.; Li, Y.; Hu, J. J. Org. Chem. 2006, 71, 6829.
[4] Prakash, G. K. S.; Chacko, S.; Alconcel, S.; Stewart, T.; Mathew, T.; Olah, G. A. Angew. Chem., Int. Ed. 2007, 46, 4933.
[5] (a) Furukawa, T.; Goto, Y.; Kawazoe, J.; Tokunaga, E.; Naka-mura, S.; Yang, Y.; Du, H.; Kakehi, A.; Shiro, M.; Shibata, N. Angew. Chem., Int. Ed. 2010, 49, 1642.
(b) Prakash, G. K. S.; Shao, N.; Zhang, Z.; Ni, C.; Wang, F.; Haiges, R.; Olah, G. A. J. Fluorine Chem. 2012, 133, 27.
(c) Shen, X.; Miao, W.; Ni, C.; Hu, J. Angew. Chem., Int. Ed. 2014, 53, 775.
[6] (a) Liu, W.-B.; Zheng, S.-C.; He, H.; Zhao, X.-M.; Dai, L.-X.; You, S.-L. Chem. Commun. 2009, 6604.
(b) Furukawa, T.; Kawazoe, J.; Zhang, W.; Nishimine, T.; Tokunaga, E.; Matsumoto, T.; Shiro, M.; Shibata, N. Angew. Chem., Int. Ed. 2011, 50, 9684.
(c) Yang, W.; Wei, X.; Pan, Y.; Lee, R.; Zhu, B.; Liu, H.; Yan, L.; Huang, K.-W.; Jiang, Z.; Tan, C.-H. Chem. Eur. J. 2011, 17, 8066.
[7] (a) Shen, X.; Zhang, L.; Zhao, Y.; Zhu, L.; Li, G.; Hu, J. Angew. Chem., Int. Ed. 2011, 50, 2588.
(b) Ma, H.; Matsuzaki, K.; Yang, Y.-D.; Tokunaga, E.; Nakane, D.; Ozawa, T.; Masuda, H.; Shibata, N. Chem. Commun. 2013, 49, 11206.
(c) Shen, X.; Ni, C.; Hu, J. Chin. J. Chem. 2013, 31, 878.
(d) Mizuta, S.; Shibata, N.; Goto, Y.; Furukawa, T.; Nakamura, S.; Toru, T. J. Am. Chem. Soc. 2007, 129, 6394.
(e) Prakash, G. K. S.; Gurung, L.; Jog, P. V.; Tanaka, S.; Thomas, T. E.; Ganesh, N.; Haiges, R.; Mathew, T.; Olah, G. A. Chem. Eur. J. 2013, 19, 3579.
[8] (a) Furukawa, T.; Shibata, N.; Mizuta, S.; Nakamura, S.; Toru, T.; Shiro, M. Angew. Chem., Int. Ed. 2008, 47, 8051.
(b) Zhang, S.; Zhang, Y.; Ji, Y.; Li, H.; Wang, W. Chem. Commun. 2009, 4886.
(c) Alba, A.-N.; Companyó, X.; Moyano, A.; Rios, R. Chem. Eur. J. 2009, 15, 7035.
(d) Moon, H. W.; Cho, M. J.; Kim, D. Y. Tetrahedron Lett. 2009, 50, 4896.
(e) Ullah, F.; Zhao, G.-L.; Deiana, L.; Zhu, M.; Dziedzic, P.; Ibrahem, I.; Hammar, P.; Sun, J.; Córdova, A. Chem. Eur. J. 2009, 15, 10013.
[9] Ni, C.; Zhang, L.; Hu, J. J. Org. Chem. 2008, 73, 5699.
[10] hen, X.; Zhang, W.; Zhang, L.; Luo, T.; Wan, X.; Gu, Y.; Hu, J. Angew. Chem., Int. Ed. 2012, 51, 6966.
[11] Ogasawara, M.; Murakami, H.; Furukawa, T.; Takahashi, T.; Shibata, N. Chem. Commun. 2009, 7366.
[12] (a) Baszczyňski, O.; Zaneba, Z. Med. Res. Rev. 2013, 33, 1304.
(b) Jindrich, J.; Holý, A.; Dvoráková, H. Collect. Czech. Chem. Commun. 1993, 58, 1645.
(c) Kiesewetter, D. O.; Knudson, K.; Collins, M.; Srinivasula, S.; Lim, E.; Mascio, M. D. J. Labelled Compd. Radiopharm. 2008, 51, 187.
[13] (a) Liang, L.; Xie, M.-S.; Wang, H.-X.; Niu, H.-Y.; Qu, G.-R.; Guo, H.-M. J. Org. Chem. 2017, 82, 5966.
(b) Liang, L.; Xie, M.-S.; Qin, T.; Zhu, M.; Qu, G.-R.; Guo, H.-M. Org. Lett. 2017, 19, 5212.
(c) Sun, H.-L.; Chen, F.; Xie, M.-S.; Guo, H.-M.; Qu, G.-R.; He, Y.-M.; Fan, Q.-H. Org. Lett. 2016, 18, 2260.
(d) Zhang, D.-J.; Xie, M.-S.; Qu, G.-R.; Gao, Y.-W.; Guo, H.-M. Org. Lett. 2016, 18, 820.
(e) Xie, M.-S.; Wang, Y.; Li, J.-P.; Du, C.; Zhang, Y.-Y.; Hao, E.-J.; Zhang, Y.-M.; Qu, G.-R.; Guo, H.-M. Chem. Commun. 2015, 51, 12451.
(f) Niu, H.-Y.; Du, C.; Xie, M.-S.; Wang, Y.; Zhang, Q.; Qu, G.-R.; Guo, H.-M. Chem. Commun. 2015, 51, 3328.
(g) Wei, T.; Xie, M.-S.; Qu, G.-R.; Niu, H.-Y.; Guo, H.-M. Org. Lett. 2014, 16, 900.
[14] For reviews about synthesis of acyclic nucleoside analogues, see:(a) Xie, M.-S.; Niu, H.-Y.; Qu, G.-R.; Guo, H.-M. Tetrahedron Lett. 2014, 55, 7156.
(b) Guo, H.-M.; Wu, S.; Niu, H.-Y.; Song, G.; Qu, G.-R. In Chemical Synthesis of Nucleoside Analogues, Ed.:Merino, P., John Wiley & Sons, Hoboken, New Jersey, 2013, p. 103.
/
| 〈 |
|
〉 |