

Chinese Journal of Organic Chemistry >
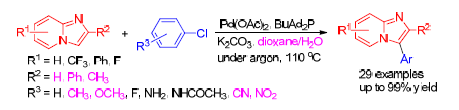
Efficient Pd-Catalyzed Direct C-H Bond Arylation of Imidazo-[1, 2-a]pyridines with Aryl Chlorides in Aqueous Medium
Received date: 2017-10-24
Revised date: 2017-11-27
Online published: 2017-11-28
Supported by
Project supported by the National Natural Science Foundation of China (No. 21702191), the Scientific and Technological Project of Henan Province (No. 172102210555) and the Postdoctoral Science Foundation of Henan Province (No. 2014003).
An efficient and practical protocol for palladium-catalyzed direct C—H bond arylation of imidazo[1,2-a]pyridines with cheap aryl/heteroaryl chlorides has been developed. Various imidazo[1,2-a]pyridines with electron-neutral, electron-poor, electron-rich, even sterically hindered aryl chlorides and heteroaryl chlorides were successfully applied to the reaction in aqueous medium to achieve the 3-arylimidazo[1,2-a]pyridines in mostly good to excellent yields, thus representing a signi?- cant advancement in the implementation of the direct C—H bond arylation of imidazo[1,2-a]pyridines with aryl chlorides.
Mu Bing , Li Jingya , Zou Dapeng , Wu Yusheng , Chang Junbiao , Wu Yangjie . Efficient Pd-Catalyzed Direct C-H Bond Arylation of Imidazo-[1, 2-a]pyridines with Aryl Chlorides in Aqueous Medium[J]. Chinese Journal of Organic Chemistry, 2018 , 38(1) : 95 -102 . DOI: 10.6023/cjoc201710029
[1] Bagdi, A. K.; Santra, S.; Monir, K.; Hajra, A. Chem. Commun. 2015, 51, 1555.
[2] Moraski, G. C.; Markley, L. D.; Hipskind, P. A.; Boshoff, H.; Cho, S.; Franzblau, S. G.; Miller, M. J. ACS Med. Chem. Lett. 2011, 2, 466.
[3] Egner, U.; Gerbling, K. P.; Hoyer, G. A.; Krüger, G.; Wegner, P. Pestic. Sci. 1996, 47, 145.
[4] Bae, J. S.; Lee, D. W.; Lee, D. H.; Jeong, D. S. WO 2007011163, 2007[Chem. Abstr. 2007, 146, 193485].
[5] El-Sayed, W. M.; Hussin, W. A.; Al-Faiyz, Y. S.; Ismail, M. A. Eur. J. Pharmacol. 2013, 715, 212.
[6] Lacerda, R. B.; de Lima, C. K. F.; da Silva, L. L.; Romeiro, N. C.; Miranda, A. L. P.; Barreiro, E. J.; Fraga, C. A. M. Bioorg. Med. Chem. 2009, 17, 74.
[7] Shukla, N. M.; Salunke, D. B.; Yoo, E.; Mutz, C. A.; Balakrishna, R.; David, S. A. Bioorg. Med. Chem. 2012, 20, 5850.
[8] Ismail, M. A.; Arafa, R. K.; Wenzler, T.; Brun, R.; Tanious, F. A.; Wilson, W. D.; Boykin, D. W. Bioorg. Med. Chem. 2008, 16, 683.
[9] Véron, J. B.; Allouchi, H.; Enguehard-Gueiffier, C.; Snoeck, R.; Andrei, G.; De Clercq, E.; Gueiffier, A. Bioorg. Med. Chem. 2008, 16, 9536.
[10] Kaminski, J. J.; Doweyko, A. M. J. Med. Chem. 1997, 40, 427.
[11] Rival, Y.; Grassy, G.; Taudon, A.; Ecalle, R. Eur. J. Med. Chem. 1991, 26, 13.
[12] Enguehard-Gueiffier, C.; Musiu, S.; Henry, N.; Véron, J. B.; Mavel, S.; Neyts, J.; Leyssen, P.; Paeshuyse, J.; Gueiffier, A. Eur. J. Med. Chem. 2013, 64, 448.
[13] Patel, H. S.; Linn, J. A.; Drewry, D. H.; Hillesheim, D. A.; Zuercher, W. J.; Hoekstra, W. J. Tetrahedron Lett. 2003, 44, 4077.
[14] Liu, G. P.; Cong, X. F.; He, J. H.; Luo, S. Z.; Wu, D.; Lan, J. B. J. Chem. Res. 2012, 36, 687.
[15] Wu, Z. Q.; Pan, Y. Y.; Zhou, X. G. Synthesis 2011, 2255.
[16] Dixon, L. I.; Carroll, M. A.; Gregson, T. J.; Ellames, G. J.; Harrington, R. W.; Clegg, W. Org. Biomol. Chem. 2013, 11, 5877.
[17] Collins, M. R.; Huang, Q.; Ornelas, M. A.; Scales, S. A. Tetrahedron Lett. 2010, 51, 3528.
[18] Monir, K.; Bagdi, A. K.; Ghosh, M.; Hajra, A. Org. Lett. 2014, 16, 4630.
[19] Enguehard, C.; Renou, J. L.; Collot, V.; Hervet, M.; Rault, S.; Gueiffier, A. J. Org. Chem. 2000, 65, 6572.
[20] Marhadour, S.; Bazin, M. A.; Marchand, P. Tetrahedron Lett. 2012, 53, 297.
[21] Nandi, D.; Jhou, Y. M.; Lee, J. Y.; Kuo, B. C.; Liu, C. Y.; Huang, P. W.; Lee, H. M. J. Org. Chem. 2012, 77, 9384.
[22] Karale, U. B.; Kalari, S.; Shivakumar, J.; Makane, V. B.; Babar, D. A.; Thakare, R. P.; Nagendra Babu, B.; Chopra, S.; Rode, H. B. RSC Adv. 2016, 6, 65095.
[23] Mu, B.; Wu, Y. S.; Li, J. Y.; Zou, D. P.; Chang, J. B.; Wu, Y. J. Org. Biomol. Chem. 2016, 14, 246.
[24] Lee, J.; Chung, J.; Byun, S. M.; Kim, B. M.; Lee, C. Tetrahedron 2013, 69, 5660.
[25] Fu, H. Y.; Chen, L.; Doucet, H. J. Org. Chem. 2012, 77, 4473.
[26] Nandi, D.; Siwal, S. S.; Mallick, K. ChemistrySelect 2017, 2, 1747.
[27] Cao, H.; Zhan, H. Y.; Lin, Y. G.; Lin, X. L.; Du, Z. D.; Jiang, H. F. Org. Lett. 2012, 14, 1688.
[28] Kalari, S.; Babar, D. A.; Karale, U. B.; Makane, V. B.; Rode, H. B. Tetrahedron Lett. 2017, 58, 2818.
[29] Choy, P. Y.; Luk, K. C.; Wu, Y. N.; So, C. M.; Wang, L. L.; Kwong, F. Y. J. Org. Chem. 2015, 80, 1457.
[30] Cao, H.; Lin, Y. G.; Zhan, H. Y.; Du, Z. D.; Lin, X. L.; Liang, Q. M.; Zhang, H. RSC Adv. 2012, 2, 5972.
[31] Liu, Q. X.; He, B. Y.; Qian, P. C.; Shao, L. X. Org. Biomol. Chem. 2017, 15, 1151.
[32] Zhao, G. K.; Zhang, K. N.; Wang, L.; Li, J. Y.; Zou, D. P.; Wu, Y. J.; Wu, Y. S. Tetrahedron Lett. 2015, 56, 6700.
[33] Ke, C. H.; Kuo, B. C.; Nandi, D.; Lee, H. M. Organometallics 2013, 32, 4775.
[34] Frett, B.; McConnell, N.; Smith, C. C.; Wang, Y. X.; Shah, N. P.; Li, H. Y. Eur. J. Med. Chem. 2015, 94, 123.
[35] Hiebel, M. A.; Fall, Y.; Scherrmann, M. C.; Berteina-Raboin, S. Eur. J. Org. Chem. 2014, 2014, 4643.
[36] Cooper, K.; Fray, M. J.; Parry, M. J.; Richardson, K.; Steele, J. J. Med. Chem. 1992, 35, 3115.
/
| 〈 |
|
〉 |