

Chinese Journal of Organic Chemistry >
Synthesis of Phosphine Ligands Based on 5-Amino-o-cresol and Its Application in Ethylene Oligomerization
Received date: 2017-06-09
Revised date: 2017-07-28
Online published: 2017-11-28
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 51534004, U1362110), and the Program for New Century Excellent Talents in University (No. NCET-07-0142).
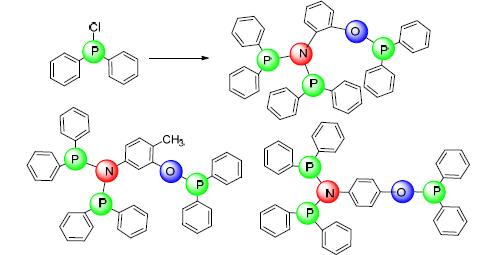
A phosphorus ligand containing PNP and P-O structure was synthesized by substitution reaction of 5-amino-o-cresol with chlorodiphenylphosphine and its structure was conformed. Its in situ prepared complex with Cr(acac)3 and preformed complex with CrCl3(THF)3 were used as main catalysts in catalyzing ethylene oligomerization, accompanied with methylaluminoxane (MAO) as cocatalyst. The effects of solvent, temperature, pressure and Al/Cr molar ratio on the activity and selectivity of the catalyst were investigated and compared with the in situ formation of 2-aminophenol and 4-aminophenol phosphine ligands catalytic system of catalyzing ethylene oligomerization effect. The experimental results showed that the activity reached 5.91×106 g/(mol·Cr·h), when the reaction was carried at 50℃ with reaction pressure of 2.5 MPa and the Al/Cr molar ratio of 700. The selectivity of 1-octene was 72.94% and the total selectivity of 1-hexene and 1-octene was 82.11%.
Feng Zhichao , Mao Guoliang , Wu Wei , Luo Mingjian , Liu Yang . Synthesis of Phosphine Ligands Based on 5-Amino-o-cresol and Its Application in Ethylene Oligomerization[J]. Chinese Journal of Organic Chemistry, 2018 , 38(3) : 698 -704 . DOI: 10.6023/cjoc201706010
[1] Skupinska, J. Chem. Rev. 1991, 91, 613.
[2] Forestière, A.; Olivier-Bourbigou, H.; Saussine, L. Oil Gas Sci. Technol. 2009, 64, 649.
[3] Qian, B. Z. Petrochem. Ind. Technol. 2011, 18, 58(in Chinese). (钱伯章, 石化技术, 2011, 18, 58.)
[4] An, J. X. Synth. Lubr. 2016, 43, 14(in Chinese). (安军信, 合成润滑材料, 2016, 43, 14.)
[5] Carter, A.; Cohen, S. A.; Cooley, N. A.; Murphy, A.; Scutt, J.; Wass, D. F. Chem. Commun. 2002, 8, 858.
[6] Bollmann, A.; Blann, K.; Dixon, J. T.; Hess, F. M.; Killian, E.; Maumela, H.; McGuinness, D. S.; Morgan, D. H.; Neveling, A.; Otto, S.; Overett, M.; Slawin, A. M. Z.; Wasserscheid, P.; Kuhlmann, S. J Am Chem Soc. 2004, 126, 14712.
[7] Varga, V.; Hodik, T.; Lamac, M.; Horacek, M.; Zukal, A.; Zilkova, N.; Parker Jr, W. O.; Pinkas, J. J. Organomet. Chem. 2015, 777, 57.
[8] Zhang, J.; Li, A.; Hor, T. S. A. Organometallics 2009, 28, 2935.
[9] Xu, J. Y. Petrol Refine Chem Ind. 2014, 45, 67(in Chinese). (许建耘, 石油炼制与化工, 2014, 45, 67.)
[10] Liu, S. L.; Yuan, W.; Luo, C. T. Aging Appl. Synth. Mater. 2016, 45, 132(in Chinese). (刘素丽, 袁炜, 罗春桃, 合成材料老化与应用, 2016, 45, 132.)
[11] Wang, J.; Li, Y.; Li, C. Q.; Zhang, H. Z. Chem. Ind. Eng Prog. 2012, 31, 91(in Chinese). (王俊, 李云, 李翠勤, 张怀志, 化工进展, 2012, 31, 91.)
[12] Wang, J.; Liang H. J.; Li, C. Q.; Shi, W. G. Chem. Ind. Eng. Prog. 2016, 35, 793(in Chinese). (王俊, 梁红姣, 李翠勤, 施伟光, 化工进展, 2016, 35, 793.)
[13] Wang, J.; Fu, Z. J.; Li, C. Q.; Shi, W. G.; Wang, S. H. Polym. Mater. Sci. Eng. 2016, 32, 176(in Chinese). (王俊, 符子剑, 李翠勤, 施伟光, 王斯晗, 高分子材料科学与工程, 2016, 32, 176.)
[14] Wang, J.; Gong, X. Y.; Li, C. Q.; Li, H. Y. Chem. Ind. Eng. Prog. 2012, 31, 2729(in Chinese). (王俊, 宫喜艳, 李翠勤, 李海燕, 化工进展, 2012, 31, 2729.)
[15] Yang, L. J.; Wang, W. Z.; Wu, Y. Chin. J. Chem. 2014, 77, 951(in Chinese). (杨磊杰, 王文珍, 吴洋, 化学通报, 2014, 77, 951.)
[16] Li, C. Q.; Lin, Z. Y.; Wang, J.; Gong, X. Y.; Zhao, Q.; Wang, Y. R.; Shao, N. Synth. Chem. 2015, 23, 198(in Chinese). (李翠勤, 林治宇, 王俊, 宫喜艳, 赵千, 王玉茹, 邵楠, 合成化学, 2015, 23, 198.)
[17] McGuinness, D. S. Chem. Rev. 2011, 111, 2321.
[18] Agapie, T. Coord. Chem. Rev. 2011, 255, 861
[19] Bryliakov, K. P.; Talsi, E. P. Coord. Chem. Rev. 2012, 256, 2994.
[20] Dixon, J. T.; Green, M. J.; Hess, F. M.; Morgan, D. H. J. Organomet. Chem. 2004, 689, 3641.
[21] Belov, G. P. Catal. Ind. 2014, 6, 266.
[22] Leeuwen, P. W. N. M. V.; Clément, N. D.; Tschan, J. L. Coord. Chem. Rev. 2011, 255, 1499.
[23] Wang, T.; Gao, X.; Shi, P.; Pei, H.; Jiang, T. Appl. Petro. Res. 2015, 5, 143.
[24] Sydora, O. L.; Jones, T. C.; Small, B. L.; Nett, A. J.; Fischer, A. A.; Carney, M. J. ACS Catal. 2012, 2, 2452.
[25] Radcliffe, J. E.; Batsanov, A. S.; Smith, D. M.; Scott, J. A.; Dyer, P. W.; Hanton, M. J. ACS Catal. 2015, 115, 7095.
[26] Liu, R.; Xiao, S. M.; Zhong, X. H.; Cao, Y. C.; Liang, S. B.; Liu, Z. Y.; Ye, X. F.; Shen, A.; Zhu, H. P. Chin. J. Org. Chem. 2015, 35, 1861(in Chinese). (刘睿, 肖树萌, 钟向宏, 曹育才, 梁胜彪, 刘振宇, 叶晓峰, 沈安, 朱红平, 有机化学, 2015, 35, 1861.)
[27] Wang, J.; Huo, H. L.; Ma, L. L.; Li, C. Q.; Shi, W. G. Chem. Bull. 2016, 79, 31(in Chinese). (王俊, 霍宏亮, 马立莉, 李翠勤, 施伟光, 化学通报, 2016, 79, 31.)
[28] Kuhlmann, S.; Dixon, J. T.; Haumann, M.; Morgan, D. H.; Ofili, J.; Spuhl, O.; Taccardi, N.; Wasserscheid, P. Adv. Synth. Catal. 2006, 348, 1200.
[29] Wang, Y. Y.; Wang, H. H.; Chuang, T. L.; Chen, B. H.; Lee, D. J. Energy Procedia. 2014, 61, 933.
[30] Overett, M. J.; Blann, L. K.; Bollmann, A.; Dixon, J. T.; Haasbroek, D.; Killian, E.; Maumela, H.; Mcguinness, D. S.; Morgan, D. H. J. Am. Chem. Soc. 2005, 127, 10723.
[31] Shao, H. Q.; Li, Y. F.; Gao, X. L.; Cao, C. G.; Tao, Y. Q.; Lin, J. C.; Jiang, T. J. Mol. Catal. A:Chem. 2014, 390, 152.
[32] Shao, H. Q.; Zhou, H.; Guo, X. Y.; Tao, Y. Q.; Jiang, T.; Qin, M. G. Catal. Commun. 2015, 60, 14.
[33] Peulecke, N.; Muller, B. H.; Spannenberg, A.; Hohne, M.; Rosenthal, U.; Wohl, A.; Muller, W.; Alqahtani. A.; Alhazmi, M. Dalton Trans. 2016, 45, 8869.
[34] Wohl, A.; Muller, W.; Peulecke, N.; Muller, B. H.; Peitz, S.; Heller, D.; Rosenthal, U. J. Mol. Catal. A:Chem. 2009, 297, 1.
[35] Song, C.; Mao, G. L.; Liu, Z. H.; Ning, Y. N.; Jiang, T. Chin. J. Org. Chem. 2016, 36, 2105(in Chinese). (宋闯, 毛国梁, 刘振华, 宁英男, 姜涛, 有机化学, 2016, 36, 2105.)
[36] Wang, S. H.; Li, J. Z.; Wang, G. Z.; Zhang, B. J.; Qu, J. B. Polym. Bull. 2012, 4, 122.
[37] Mogorosi, M. M.; Mahamo, T.; Moss, J. R.; Mapolie, S. F.; Slootweg, J. C.; Lammertsma, K.; Smith, G. S. J. Organomet. Chem. 2011, 696, 3585.
[38] Tang, X. B.; Zhang, D. H.; Jie, S. Y.; Sun, W. H.; Chen, J. T. J. Organomet. Chem. 2005, 690, 3918.
[39] Jabri, A.; Temple, C.; Crewdson, P.; Gambarotta, S.; Korobkov, L.; Duchateau, R. J. Am. Chem. Soc. 2006, 128, 9238.
[40] McGuinness, D. S.; Rucklidge, A. J.; Tooze, R. P.; Slawin, A. M. Z. Organometallics 2007, 26, 2561.
[41] Walsh, R.; Morgan, D. H.; Bollmann, A.; Dixon J. T. Appl. Catal. A:Gen. 2006, 306, 184.
[42] Chen, J. X.; Huang, Y. B.; Li, Z. S.; Zhang, Z. C.; Wei, C. X.; Lan, T. Y.; Zhang, W. J. J. Mol. Catal. A:Chem. 2006, 259, 133.
[43] Sun, W. H.; Song, S. J.; Li, B. X.; Redshaw, C.; Hao, X.; Li, Y. S.; Wang, F. S. Dalton Trans. 2012, 41, 11999.
[44] Ning, Y. N.; Niu, B.; Jiang, T.; Ding, W. Y.; Yin, X. F. Chem. Prod. Technol. 2009, 16, 19(in Chinese). (宁英男, 牛博, 姜涛, 丁万友, 殷喜丰, 化工生产与技术, 2009, 16, 19.)
[45] Ning, Y. N.; Xue, Q. M.; Mao, G. L.; Jiang, T. Chem. Prog. 2011, 30, 1003(in Chinese). (宁英男, 薛秋梅, 毛国梁, 姜涛, 化工进展, 2011, 30, 1003.)
[46] Sa, S.; Lee, S. M.; Sang, Y. K. J. Mol. Catal. A Chem. 2013, 378, 17.
[47] Mao, G. L.; Ning, Y. N.; Hu, W. B.; Li, S. M.; Song, X. F.; Niu, B.; Jiang, T. Chin. Sci. Bull. 2008, 53, 3511.
[48] Wang, Q. A.; Fan, H. F.; Liao, T. G. Technical Handbook of Organic Chemistry Laboratory, Chemical Industry Press, Beijing, 2012, pp. 201~205(in Chinese). (王秋安, 范华芳, 廖头根, 有机化学实验室技术手册, 化学工业出版社, 北京, 2012, pp. 201~205.)
/
| 〈 |
|
〉 |