

Chinese Journal of Organic Chemistry >
Design and Synthesis of Natural Product Mevalocidin Chiral Center Based Analogues
Received date: 2017-10-21
Revised date: 2017-11-24
Online published: 2017-12-15
Supported by
Project supported by the National Natural Science Foundation of China (No. 21472063).
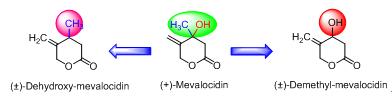
Mevalocidin is a phytotoxin, produced by two fungal isolates designated Rosellinia DA092917 and Fusarium DA056446, and has a broad spectrum of post emergence herbicidal activity at relatively high rate (4 kg/ha). Structural modification of mevalocidin at its chiral center was investigated with the aim of discovering of novel herbicides with improved activity. In order to determine the role of hydroxyl and methyl groups of mevalocidin exhibiting the herbicidal activity, we designed (±)-deoxy-mevalocidin (1) and (±)-demethyl-mevalocidin (2), respectively. These two designed compounds were synthesized (racemate) through two different synthetic approaches and characterized by 1H NMR, 13C NMR and HRMS spectra. The herbicidal activities of the synthesized compounds were evaluated against both dicotyledon and monocotyledon weeds. Bioassay results indicated that the both synthesized analogues lost the herbicidal activity when compared to parent and it demonstrated that methyl and/or hydroxyl groups at the chiral center of mevalocidin are crucial for its herbicidal activity. Thus, this study will provide insights into the structural modification of mevalocidin.
Wu Qiongyou , Zhang Rui , Pan Jinhuan , Clough John , Gu Yucheng , Yang Guangfu . Design and Synthesis of Natural Product Mevalocidin Chiral Center Based Analogues[J]. Chinese Journal of Organic Chemistry, 2018 , 38(4) : 840 -845 . DOI: 10.6023/cjoc201710023
[1] Guan, A. Y.; Liu, C. L.; Yang, X. P.; Dekeyser, M. Chem. Rev. 2014, 114, 7079.
[2] Hashidoko, Y.; Shinano, T. J. Pest. Sci. 2011, 36, 106.
[3] Jeschke, P. Pest Manage. Sci. 2010, 66, 10.
[4] Damalas, C. A.; Eleftherohorinos, I. G. Int. J. Environ. Res. Public Health 2011, 8, 1402.
[5] Duke, S. O. Pest Manage. Sci. 2012, 68, 505.
[6] Dayan, F. E.; Owens, D. K.; Duke, S. O. Pest Manage. Sci. 2012, 68, 519.
[7] Butler, M. S. J. Nat. Prod. 2004, 67, 2141.
[8] Dayan, F. E.; Duke, S. O. Plant Physiol. 2014, 166, 1090.
[9] Zhang, Z.; Xing, A.; Staswick, P. Clemente, T. E. Plant Cell, Tissue Organ Cul. 1999, 56, 37.
[10] Bayer, E.; Gugel, K. H.; Haegele, K.; Hagenmaier, H.; Esipov, S. E.; Koenig, W. A.; Zaehner, H. Helv. Chim. Acta 1972, 55, 224.
[11] Omura, S.; Hinotozawa, K.; Imamura, N.; Murata, M. J. Antibiot. 1984, 37, 939.
[12] Hoerlein, G. Rev. Environ. Contam. Toxicol. 1994, 138, 73.
[13] Mitchell, G.; Bartlett, D. W.; Fraser, T. E. M.; Hawkes, T. R.; Holt, D. C.; Townson, J. K.; Wichert, R. A. Pest Manage. Sci. 2001, 57, 120.
[14] Gerwick, B. C.; Graupner, P. R.; Fields, S. C.; Schmitzer, P. R.; Brewster, W. K. US 7393812, 2008[Chem. Abstr. 2006, 485213].
[15] Lichtner, F. Austral. J. Plant Physiol. 2000, 27, 609.
[16] Gerwick, B. C.; Brewser, W. K.; de Bore, G. J.; Fields, S. C.; Graupner, P. R.; Hahn, D. R.; Pearce, C. J.; Schmitzer, P. R.; Webster, J. D. J. Chem. Ecol. 2013, 39, 253.
[17] Lin, L.; Mulholland, N.; Wu, Q. Y.; Beattie, D.; Huang, S. W.; Irwin, D.; Clough, J.; Gu, Y. C.; Yang, G. F. J. Agric. Food Chem. 2012, 60, 4480.
[18] Lin, L.; Mulholland, N.; Huang, S. W.; Beattie, D.; Irwin, D.; Gu, Y. C.; Clough, J.; Wu, Q. Y.; Yang, G. F. Chem. Biol. Drug Des. 2012, 80, 682.
[19] Larsen, C. H.; Ridgway, B. H.; Shaw, J. T.; Woerpel, K. A. J. Am. Chem. Soc. 1999, 121, 12208.
[20] Villieras, J.; Rambaud, M. Synth. Commun. 1983, 300.
[21] Suizu, H.; Shigeoka, D.; Aoyama, H.; Yoshimitsu, T. Org. Lett. 2015, 17, 126.
/
| 〈 |
|
〉 |