

Chinese Journal of Organic Chemistry >
Synthesis and Cell Division Cycle 25B Phosphatase/Protein Tyrosine Phosphatase 1B Inhibitory Activity Evaluation of Novel Acylthiourea Derivatives
Received date: 2017-09-13
Revised date: 2017-12-04
Online published: 2018-01-03
Supported by
Project supported by the Natural Science Foundation of Liaoning Province (No. 20102126).
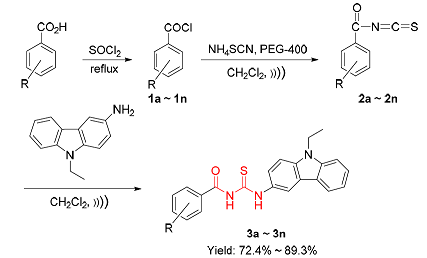
A series of new acylthiourea derivatives 3 containing carbazole moity have been synthesized by the techniques of ultrasonic irradiation and solid-liquid phase transfer catalysis. Their structures were characterized by IR, 1H NMR, 13C NMR spectra and elemental analysis. This synthetic method has the advantages of short reaction time, simple operation and high yield. All synthesized target compounds were screened for their inhibitory activity against cell division cycle 25B phosphatase (Cdc25B) and protein tyrosine phosphatase 1B (PTP1B). The results show that all the compounds 3 display significant inhibitory activities against Cdc25B, and partial target compounds 3 also show significant inhibitory activities against PTP1B. Among them, 1-(4-nitrobenzoyl)-3-(9-ethyl-carbazole-3-yl)thiourea (3n) exhibits highest inhibitory activity against Cdc25B [IC50=(0.49±0.12) mg/mL] and 1-(2-nitrobenzoyl)-3-(9-ethyl-carbazole-3-yl)thiourea (3l) displays highest inhibitory activity against PTP1B [IC50=(3.59±1.15) mg/mL]. It is noteworthy that compound 3n shows higher inhibitory activity against Cdc25B and PTP1B. The preliminary research results of molecular docking revealed the structural-activity of the inhibitors. The active compounds can be considered as potential Cdc25B and PTP1B inhibitors, and have great application prospects in the treatment of cancers and diabetes.
Key words: acylthiourea; carbazole; synthesis; Cdc25B and PTP1B inhibitors; molecular docking
Li Yingjun , Wang Siyuan , Jin Kun , Gao Lixin , Sheng Li , Zhang Nan , Yang Kaidong , Zhao Yue , Li Jian . Synthesis and Cell Division Cycle 25B Phosphatase/Protein Tyrosine Phosphatase 1B Inhibitory Activity Evaluation of Novel Acylthiourea Derivatives[J]. Chinese Journal of Organic Chemistry, 2018 , 38(5) : 1242 -1250 . DOI: 10.6023/cjoc201709022
[1] Nitulescu, G. M.; Draghici, C.; Olaru, O. T.; Matei, L.; Ioana, A.; Dragu, L. D.; Bleotu, C. Bioorg. Med. Chem. 2015, 23, 5799.
[2] Jin, L.; Qu, H. E.; Huang, X. C.; Pan, Y. M.; Liang, D.; Chen, Z. F.; Wang, H. S.; Zhang, Y. Int. J. Mol. Sci. 2015, 16, 14571.
[3] Yun, T.; Qin, T.; Liu, Y.; Lai, L. H. Eur. J. Med. Chem. 2016, 124, 229.
[4] Gunasekaran, N.; Vadivel, V.; Halcovitch, N. R.; Tiekink, E. R. T. Chem. Data Collect. 2017, 9, 263.
[5] Kulabas, N.; Özakpinar, Ö. B.; Özsavci, D.; Leyssen, P.; Neyts, J.; Küçükgüzel, I. Marmara Pharm. J. 2017, 21, 371.
[6] Banaei, A.; Shiran, J. A.; Saadat, A.; Ardabili, F. F.; McArdle, P. J. Mol. Struct. 2015, 1099, 427.
[7] Tahir, S.; Badshah, A.; Hussain, R. A.; Tahir, M. N.; Tabassum, S.; Patujo, J. A.; Rauf, M. K. J. Mol. Struct. 2015, 1099, 215.
[8] Cui, P. L.; Li, X. L.; Zhu, M. Y.; Wang, B. H.; Liu, J.; Chen, H. Bioorg. Med. Chem. Lett. 2017, 27, 2234.
[9] Zullkiplee, W. S. H. W.; Ariff, M. A. M.; Hussain, H.; Khairul W. M.; Ngaini, Z. Phosphorus, Sulfur Silicon Relat. Elem. 2016, 191, 1329.
[10] Khairul, W. M.; Ariffin, A. A.; Ismail, N.; Daud, A. I. Educ. Jsmt. 2016, 3, 13.
[11] Halim, A. N. A.; Ngaini, Z. Phosphorus, Sulfur Silicon Relat. Elem. 2017, 192, 1.
[12] Sun, C. W.; Huang, H.; Feng, M. Q.; Shi, X. L.; Zhang, X. D.; Zhou, P. Bioorg. Med. Chem. Lett. 2006, 16, 162.
[13] Burgeson, J. R.; Moore, A. L.; Boutilier, J. K.; Cerruti, N. R.; Gharaibeh, D. N.; Lovejoy, C. E.; Amberg, S. M.; Hruby, D. E.; Tyavanagimatt, S. R.; Allen Ⅲ, R. D.; Dai, D. C. Bioorg. Med. Chem. Lett. 2012, 22, 4263.
[14] Dobrikov, G. M.; Valcheva, V.; Nikolova, Y.; Ugrunova, I.; Pasheva, E.; Dimitrov, V. Eur. J. Med. Chem. 2013, 63, 468.
[15] Saeed, A.; Shah, M. S.; Larik, F. A.; Khan, S. U.; Channar, P. A.; Flörke, U.; Iqbal, J. Med. Chem. Res. 2017, 26, 1635.
[16] Wang, M. J.; Nan, X.; Feng, G.; Yu, H. T.; Hu, G. F.; Liu, Y.-Q. Ind. Crop. Prod. 2014, 55, 11.
[17] Chang, Y. N.; Zhang, J. W.; Chen, X. L..; Li, Z.; Xu, X. Y. Bioorg. Med. Chem. Lett. 2017, 27, 2641.
[18] Zhang, Q.; Zhao, B. H.; Song, Y. Y.; Hua, C. W.; Gou, X. F.; Chen B.; Zhao, J. L. Heteroat. Chem. 2015, 26, 348.
[19] Saeed, A.; Qamar R.; Fattah T. A.; Flörke, U.; Erben, M. F. Res. Chem. Intermed. 2017, 43, 3053.
[20] Murali, K.; Sparkes, H. A.; Prasad, K. J. R. Eur. J. Med. Chem. 2017, 128, 319.
[21] Sun, L. Q.; Wu, Y. B.; Liu, Y. H.; Chen, X. F.; Hu, L. X. Bioorg. Med. Chem. Lett. 2017, 27, 261.
[22] Dineshkumar, B.; Mitra, A.; Mahadevappa, M. Int. J. Phytomed. 2010, 2, 22.
[23] Wang, G. C.; Wang, J.; He, D. X.; Li, J.; Peng, Z. Y. Bioorg. Med. Chem. Lett. 2016, 26, 2806.
[24] Kong, X. Q.; Zhang, H. Z.; Cao, C. S.; Zhou, S. L.; Pang, G. S.; Shi, Y. H. Bioorg. Med. Chem. 2016, 24, 1376.
[25] Bandgar, B. P.; Adsul, L. K.; Chavan, H. V.; Jalde, S. S.; Shringare, S. N.; Shaikh, R.; Meshram, R. J.; Gacche, R. N.; Masand, V. Bioorg. Med. Chem. Lett. 2012, 22, 2539.
[26] Zhu, D. Q.; Chen, M. H.; Li, M.; Luo, B. L.; Zhao, Y.; Huang, P.; Xue, F. T.; Rapposelli, S.; Pi, R. B.; Wen, S. J. Eur. J. Med. Chem. 2013, 68, 81.
[27] Hieda, Y.; Anraku, M; Choshi, T.; Tomida, H.; Fujioka, H.; Hatae, N.; Hori, O.; Hirose, J.; Hibino, S. Bioorg. Med. Chem. Lett. 2014, 24, 3530.
[28] Börger, C.; Brütting, C.; Julich-Fruner, K. K.; Hesse, R.; Kumar, V. P.; Kutz, S. K.; Rönnefahrt, M.; Thomas, C.; Wan, B. J.; Franzblau, S. G.; Knölker, H. J. Bioorg. Med. Chem. 2017, 25(22), 6167.
[29] Ma, Q. G.; Tian, J.; Yang, J. B.; Wang, A. G.; Ji, T. F.; Wang, Y. G.; Su, Y. L. Fitoterapia 2013, 87, 1.
[30] Ty, N.; Dupeyre, G.; Chabot, G. G.; Seguin, J.; Quentin, L.; Chiaroni, A.; Tillequin, F.; Scherman, D.; Michel, S.; Cachet, X.. Eur. J. Med. Chem. 2010, 45, 3726.
[31] Akué-Gédu, R.; Nauton, L.; Théry, V.; Bain, J.; Cohen, P.; Anizon, F.; Moreau, P. Bioorg. Med. Chem. 2010, 18, 6865.
[32] Suchaud, V.; Gavara, L.; Saugues, E.; Nauton, L.; Théry, V.; Anizon, F.; Moreau, P. Bioorg. Med. Chem. 2013, 21, 4102.
[33] Lampropoulou, E.; Manioudaki, M.; Fousteris, M.; Koutsourea, A.; Nikolaropoulos, S.; Papadimitriou, E. Biomed. Pharmacother. 2011, 65, 142.
[34] Yoon, H. J.; Kong, S. Y.; Park, M. H.; Cho, Y. S.; Kim, S. E.; Shin, J. Y.; Jung, S. H.; Lee, J.; Farhanullah; Kim, H. J.; Lee, J. Bioorg. Med. Chem. 2013, 21, 7165.
[35] Thiratmatrakul, S.; Yenjai, C.; Waiwut, P.; Vajragupta, O.; Reubroycharoen, P.; Tohda, M.; Boonyarat, C. Eur. J. Med. Chem. 2014, 75, 21.
[36] Rosenker, K. M. G.; Paquette, W. D.; Johnston, P. A.; Sharlow, E. R.; Vogt, A.; Bakan, A.; Lazo, J. S.; Wipf, P. Bioorg. Med. Chem. 2015, 23, 2810.
[37] Li, Y.-J.; Yu, Y.; Jin, K.; Gao, L.-X.; Luo, T.-C..; Sheng, L.; Shao, X.; Li, J. Bioorg. Med. Chem. Lett. 2014, 24, 4125.
[38] Vo, Q. H.; Nguyen, P. H.; Zhao, B. T.; Ali, M. Y.; Choi, J. S.; Min, B. S.; Nguyen, T. H.; Woo, M. H. Fitoterapia 2015, 103, 113.
[39] Mei, W.-W.; Guo, Y.-W.; Li, J.; Cai, M.-Y.; Ma, W.-Q.; Gong, J.-X.; Wang, X.-D. Chin. J. Org. Chem. 2016, 36, 533(in Chinese). (梅雯雯, 郭跃伟, 李佳, 蔡妹艺, 马文泉, 龚景旭, 王学东, 有机化学, 2016, 36, 533.)
[40] Li, Y.-J.; Shi, X.-L.; Gao, L.-X.; Jin, K.; Sheng, L.; Wu, J.-H.; Peng, L.-N.; Li, J. Chin. J. Org. Chem. 2015, 35, 191(in Chinese). (李英俊, 史相玲, 高立信, 靳焜, 盛丽, 吴疆红, 彭丽娜, 李佳, 有机化学, 2015, 35, 191.)
[41] Li, Y.-J.; Yu, Y.; Jin, K.; Gao, L.-X.; Luo, T.-C.; Sheng, L.; Sao. X.; Li, J. Chin. J. Org. Chem. 2015, 35, 129(in Chinese). (李英俊, 于洋, 靳焜, 高立信, 罗潼川, 盛丽, 邵昕, 李佳, 有机化学, 2015, 35, 129.)
[42] Li, Y.-J.; Li, J.-Y.; Peng, L.-N.; Gao, L.-X.; Jin, K.; Sheng, L.; Zhang, N.; Wang, S.-Y.; Li, J. Chin. J. Org. Chem. 2017, 37, 485(in Chinese). (李英俊, 李继阳, 彭丽娜, 高立信, 靳焜, 盛丽, 张楠, 王思远, 李佳, 有机化学, 2017, 37, 485.)
[43] Wei, T.-B.; Chen, J.-C.; Wang, X.-C.; Yang, S.-Y. Chem. J. Chin. Univ. 1992, 13, 1217(in Chinese). (魏太保, 陈继畴, 王秀春, 杨素铀, 高等学校化学学报, 1992, 13, 1217.)
[44] Okuniewski, A.; Rosiak, D.; Chojnacki, J.; Becker, B. Acta Crystallogr. 2017, 73, 52.
[45] Saeed, A.; Ashraf, Z.; Erben, M. F.; Simpson, J. J. Mol. Struct. 2017, 1129, 283.
[46] Tabka, T.; Héron, J. F.; Gauduchon, P.; Le Talaer, J. Y.; Lancelot, J. C.; Rault, S.; Robba, M. Eur. J. Med. Chem. 1988, 23, 119.
[47] Huang, W. G.; Jiang, Y. Y.; Li, Q.; Li, J.; Li, J. Y.; Lu, W.; Cai, J. C. Tetrahedron 2005, 61, 1863.
[48] Sun, L. P.; Shen, Q.; Piao, H. H.; Ma, W. P.; Gao, L. X.; Zhang, W.; Nan, F. J.; Li, J.; Piao, H. R. Eur. J. Med. Chem. 2011, 46, 3630.
[49] Ge, Y. S.; Kamp, M. V. D.; Malaisree, M.; Liu, D.; Liu, Y.; Mulholland, A. J. J. Comput.-Aided Mol. Des. 2017, 31, 995.
[50] Wiesmann, C.; Barr, K. J.; Kung, J.; Zhu, J.; Erlanson, D. A.; Shen, W.; Fahr, B. J.; Zhong, M.; Taylor, L.; Randal, M.; McDowell, R. S.; Hansen, S. K. Nat. Struct. Mol. Biol. 2004, 11, 730.
/
| 〈 |
|
〉 |