

Chinese Journal of Organic Chemistry >
Catalytic Nucleophilic Addition of 3, 5-Dialkyl-4-nitroisoxazoles to Trifluoromethyl Ketones on Water
Received date: 2017-09-29
Revised date: 2017-12-29
Online published: 2018-01-26
Supported by
Project supported by the National Natural Sciences Foundation of China (Nos. 21402116, 21502111, 21572126), the Key Scientific and Technological Project of Henan Province (No. 172102210099) and the Key Science Research of Education Committee in Henan Province (No. 15A150072).
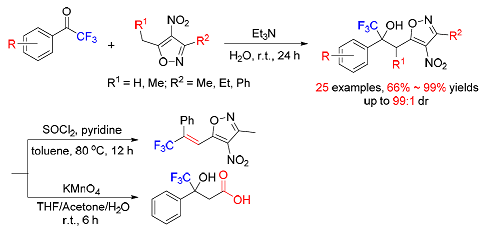
The triethylamine catalyzed nucleophilic addition of 3, 5-dialkyl-4-nitroisoxazoles to trifluoromethyl ketones on water has been realized affording trifluoromethyl tertiary alcohol derivatives in 66%~99% yields. The products were easily transformed to the resulting alkenes by dehydration or acids by oxidation.
Wang Jingjing , Li Feng , Xu Yan , Wang Juan , Wu Ziyan , Yang Chengyu , Liu Lantao . Catalytic Nucleophilic Addition of 3, 5-Dialkyl-4-nitroisoxazoles to Trifluoromethyl Ketones on Water[J]. Chinese Journal of Organic Chemistry, 2018 , 38(5) : 1155 -1164 . DOI: 10.6023/cjoc201709049
[1] (a) Welch, J. T.; Eswarakrishman, S. Fluorine in Bioorganic Chemistry, Wiley, New York, 1991.
(b) Filler, R.; Kobayashi, Y.; Yagupolskii, L. M. Organofluorine Compounds in Medicinal Chemistry and Biomedical Applications, Elsevier, Amsterdam, 1993.
(c) Banks, R. E.; Smart, B. E.; Tatlow, C. J. Organofluorine Chemistry:Principles and Comercial Applications, Springer, New York, 1994.
(d) Hiyama, T.; Kanie, K.; Kusumoto, T.; Morizawa, Y.; Shimizu, M. Organofluorine Compounds:Chemistry and Applications, Springer-Verlag, Berlin, 2000.
(e) Kirsch, P. Modern Fluoroorganic Chemistry, Wiley-VCH, Weinheim, 2005.
(f) Uneyama, K. Organofluorine Chemistry, Blackwell, Oxford, 2007.
(g) Ojima, I. Fluorine in Medicinal Chemistry and Chemical Biology, Wiley-Blackwell, Chichester, 2009.
[2] (a) Kirk, K. L. J. Fluorine Chem. 2006, 127, 1013.
(b) Mîller, K.; Faeh, C.; Diederich, F. Science 2007, 317, 1881.
(c) Purser, S.; Moore, P. R.; Swallow, S.; Gouverneur, V. Chem. Soc. Rev. 2008, 37, 320.
(d) Hagmann, W. K. J. Med. Chem. 2008, 51, 4359.
(e) Berger, R.; Resnati, G.; Metrangolo, P.; Weber, E.; Hulliger, J. Chem. Soc. Rev. 2011, 40, 3496.
(f) Ojima, I. J. Org. Chem. 2013, 78, 6358.
(g) Fujiwara, T.; O_Hagan, D. J. Fluorine Chem. 2014, 167, 16.
(h) Wang, J.; Sánchez-Roselló, M.; Aceña, J. L.; Pozo, C.; Sorochinsky, A. E.; Fustero, S.; Soloshonok, V. A.; Liu, H. Chem. Rev. 2014, 114, 2432.
(i) Gillis, E. P.; Eastman, K. J.; Hill, M. D.; Donnelly, D. J.; Meanwell, N. A. J. Med. Chem. 2015, 58, 8315.
[3] (a) Pierce, M. E.; Rodney, L. P. J.; Radesca, L. A.; Lo, Y. S.; Silverman, S.; Moore, J. R.; Islam, Q.; Choudhury, A.; Fortunak, J. M. D.; Nguyen, D.; Luo, C.; Morgan, S. J.; Davis, W. P.; Confalone, P. N.; Chen, C.-Y.; Tillyer, R. D.; Frey, L.; Tan, L.; Xu, F.; Zhao, D.; Thompson, A. S.; Corley, E. G.; Grabowski, E. J. J.; Reamer, R.; Reider, P. J. J. Org. Chem. 1998, 63, 8536.
(b) Corbett, J. W.; Ko, S. S.; Rodgers, J. D.; Gearhart, L. A.; Magnus, N. A.; Bacheler, L. T.; Diamond, S.; Jeffrey, S.; Klabe, R. M.; Cordova, B. C.; Garber, S.; Logue, K.; Trainor, G. L.; Anderson, P. S.; Erickson-Viitanen, S. K. J. Med. Chem. 2000, 43, 2019.
[4] Schenck, H. A.; Lenkowski, P. W.; Choudhury-Mukherjee, I.; Ko, S.-H.; Stables, J. P.; Patel, M. K.; Brown, M. L. Bioorg. Med. Chem. 2004, 12, 979.
[5] Fandrick, D. R.; Reeves, J. T.; Bakonyi, J. M.; Nyalapatla, P. R.; Tan, Z.; Niemeier, O.; Akalay, D.; Fandrick, K. R.; Wohlleben, W.; Ollenberger, S.; Song, J. J.; Sun, X.; Qu, B.; Haddad, N.; Sanyal, S.; Shen, S.; Ma, S.; Byrne, D.; Chitroda, A.; Fuchs, V.; Narayanan, B. A.; Grinberg, N.; Lee, H.; Yee, N.; Brenner, M.; Senanayake, C. H. J. Org. Chem. 2013, 78, 3592.
[6] (a) Betageri, R.; Zhang, Y.; Zindell, R. M.; Kuzmich, D.; Kirrane, T. M.; Bentzien, J.; Cardozo, M.; Capolino, A. J.; Fadra, T. N.; Nelson, R. M.; Paw, Z.; Shih, D.-T.; Shih, C.-K.; Zuvela-Jelaska, L.; Nebozny, G.; Thomson, D. Bioorg. Med. Chem. Lett. 2005, 15, 4761.
(b) Barker, M.; Clackers, M.; Copley, R.; Demaine, D. A.; Humphreys, D.; Inglis, G. G. A.; Johnston, M. J.; Jones, H. T.; Haase, M. V.; House, D.; Loiseau, R.; Nisbet, L.; Pacquet, F.; Skone, P. A.; Shanahan, S. E.; Tape, D.; Vinader, V. M.; Washington, M.; Uings, I.; Upton, R.; McLay, I. M.; Macdonald, S. J. F. J. Med. Chem. 2006, 49, 4216.
[7] (a) Sani, M.; Viani, F.; Binda, M.; Zaffaroni, N.; Zanda, M. Lett. Org. Chem. 2005, 2, 447.
(b) Betageri, R.; Gilmore, T.; Kuzmich, D.; Kirrane, T. M.; Bentzien, J.; Wiedenmayer, D.; Bekkali, Y.; Regan, J.; Berry, A.; Latli, B.; Kukulka, A. J.; Fedra, T. N.; Nelson, R. N.; Goldrick, S.; Zuvela-Jelaska, L.; Souza, D.; Pelletier, J.; Dinallo, R.; Panzenbeck, M.; Torcellini, C.; Lee, H.; Pack, E.; Harcken, C.; Nabozny, G.; Thomson, D. S. Bioorg. Med. Chem. Lett. 2011, 21, 6842.
[8] Carceller, E.; Merlos, M.; Giral, M.; Balsa, D.; Garciía-Rafanell, J.; Forn, J. J. Med. Chem. 1996, 39, 487.
[9] For reviews, see:(a) Nie, J.; Guo, H.-C.; Cahard, D.; Ma, J.-A. Chem. Rev. 2011, 111, 455.
(b) Kelly, C. B.; Mercadante, M. A.; Leadbeater, N. E. Chem. Commun. 2013, 49, 11133.
[10] For selected examples:(a) Hara, N.; Tamura, R.; Funahashi, Y.; Nakamura, S. Org. Lett. 2011, 13, 1662.
(b) Zhang, G.-W.; Meng, W.; Ma, H.; Nie, J.; Zhang, W.-Q.; Ma, J.-A. Angew. Chem., Int. Ed. 2011, 50, 3538.
(c) Cui, H.-F.; Wang, L.; Yang, L.-J.; Nie, J.; Zheng, Y.; Ma, J.-A. Tetrahedron 2011, 67, 8470.
(d) Li, X.-J.; Xiong, H.-Y.; Hua, M.-Q.; Nie, J.; Zheng, Y.; Ma, J.-A. Tetrahedron Lett. 2012, 53, 2117.
(e) Zheng, Y.; Xiong, H.-Y.; Nie, J.; Hua, M.-Q.; Ma, J.-A. Chem. Commun. 2012, 48, 4308.
(f) Luo, R.; Li, K.; Hu, Y.; Tang, W. Adv. Synth. Catal. 2013, 355, 1297.
(g) Zong, H.; Huang, H.; Bian, G.; Song, L. J. Org. Chem. 2014, 79, 11768.
(h) Wang, L.; Liu, N.; Dai, B.; Ma, X.; Shi, L. RSC Adv. 2015, 5, 10089.
(i) Jamal, Z.; Teo, Y.-C. RSC Adv. 2015, 5, 26949.
(j) Zhang, D.; Tanaka, F. Adv. Synth. Catal. 2015, 357, 3458.
(k) Tao, R.; Yin, X.-J.; Wang, K.-H.; Niu, Y.-Z.; Wang, Y.-L.; Huang, D.-F.; Su, Y.-P.; Wang, J.-X.; Hu, Y.-L.; Fu, Y.; Du, Z.-Y. Chin. Chem. Lett. 2015, 26, 1046.
(l) Jing, Z.; Bai, X.; Chen, W.; Zhang, G.; Zhu, B.; Jiang, Z. Org. Lett. 2016, 18, 260.
(m) Lutete, L. M.; Miyamoto, T.; Ikemoto, T. Tetrahedron Lett. 2016, 57, 1220.
(n) Cook, A. M.; Wolf, C. Angew. Chem., Int. Ed. 2016, 55, 2929.
(o) Lee, K.; Silverio, D. L.; Torker, S.; Robbins, D. W.; Haeffner, F.; Mei, F. W.; Hoveyda, A. H. Nat. Chem. 2016, 8, 768.
(p) Matador, E.; Monge, D.; Fernández, R.; Lassaletta, J. M. Green Chem. 2016, 18, 4042.
(q) Mszar, N. W.; Mikus, M. S.; Torker, S.; Haeffner, F.; Hoveyda, A. H. Angew. Chem., Int. Ed. 2017, 56, 8736.
(r) Noda, H.; Amemiya, F.; Weidner, K.; Kumagai, N.; Shibasaki, M. Chem. Sci. 2017, 8, 3260.
(s) Bai, X.; Zeng, G.; Shao, T.; Jiang, Z. Angew. Chem., Int. Ed. 2017, 56, 3684.
(t) Zheng, Y.; Tan, Y.; Harms, K.; Marsch, M.; Riedel, R.; Zhang, L.; Meggers, E. J. Am. Chem. Soc. 2017, 139, 4322.
[11] Bandini, M.; Sinisi, R. Org. Lett. 2009, 11, 2093.
[12] Zhang, Y.; Wei, B.-W.; Zou, L.-N.; Kang, M.-L.; Luo, H.-Q.; Fan, X.-L. Tetrahedron 2016, 72, 2472.
[13] Czerwinski, P.; Molga, E.; Cavallo, L.; Poater, A.; Michalak, M. Chem. Eur. J. 2016, 22, 8089.
[14] (a) Grieco, P. A. Organic Synthesis in Water, Blackie, London, 1998.
(b) Lindstrom, U. M. Organic Reactions in Water:Principles, Strategies and Applications, Blackwell, Oxford, U. K., 2007.
(c) Li, C.-J.; Chan, T.-H. Comprehensive Organic Reactions in Aqueous Media, Wiley, New York, 2007.
[15] For selected reviews:(a) Lindstrom, U. M. Chem. Rev. 2002, 102, 2751.
(b) Head-Gordon, T.; Hura, G. Chem. Rev. 2002, 102, 2651.
(c) Hayashi, Y. Angew. Chem., Int. Ed. 2006, 45, 8103.
(d) Mase, N.; Barbas Ⅲ, C. F. Org. Biomol. Chem. 2010, 8, 4043.
[16] For selected reviews:(a) Li, C.-J. Chem. Rev. 2005, 105, 3095.
(b) Li, C.-J.; Chen, L. Chem. Soc. Rev. 2006, 35, 68.
(c) Chanda, A.; Fokin, V. V. Chem. Rev. 2009, 109, 725.
(d) Butler, R. N.; Coyne, A. G. Chem. Rev. 2010, 110, 6302.
(e) Simon, M.-O.; Li, C.-J. Chem. Soc. Rev. 2012, 41, 1415.
(f) Gawande, M. B.; Bonifacio, V. D. B.; Luque, R.; Branco, P. S.; Varma, R. S. Chem. Soc. Rev. 2013, 42, 5522.
(g) Levin, E.; Ivry, E.; Diesendruck, C. E.; Lemcoff, N. G. Chem. Rev. 2015, 115, 4607.
(h) Butler, R. N.; Coyne, A. G. Org. Biomol. Chem. 2016, 14, 9945.
[17] For selected examples:(a) Phippen, C. B. W.; Beattie, J. K.; McErlean, C. S. P. Chem. Commun. 2010, 46, 8234.
(b) Fu, X.-P.; Liu, L.; Wang, D.; Chen, Y.-J.; Li, C.-J. Green. Chem. 2011, 13, 549.
(c) Sakakura, A.; Koshikari, Y.; Akakura, M.; Ishihara, K. Org. Lett. 2011, 14, 30.
(d) Paladhi, S.; Chauhan, A.; Dhara, K.; Tiwari, A. K.; Dash, J. Green. Chem. 2012, 14, 2990.
(e) Islam, S.; Larrosa, I. Chem. Eur. J. 2013, 19, 15093.
(f) Sengoden, M.; Punniyamurthy, T. Angew. Chem. Int. Ed. 2013, 52, 572.
(g) Paladhi, S.; Bhati, M.; Panda, D.; Dash, J. J. Org. Chem. 2014, 79, 1473.
(h) Thakur, P. B.; Meshram, H. M. RSC Adv. 2014, 4, 5343.
(i) Thakur, P. B.; Meshram, H. M. RSC Adv. 2014, 4, 6019.
(j) Yu, J.-S.; Liu, Y.-L.; Tang, J.; Wang, X.; Zhou, J. Angew. Chem. Int. Ed. 2014, 53, 9512.
(k) Yang, F.; Klumphu, P.; Liang, Y.-M.; Lipshutz, B. H. Chem. Commun. 2014, 50, 936.
(l) Zhang, F.-Z.; Tian, Y.; Li, G.-X.; Qu, J. J. Org. Chem. 2015, 80, 1107.
(m) SaiPrathima, P.; Srinivas, K.; Mohan Rao, M. Green. Chem. 2015, 17, 2339.
(n) Bhattacharjya, A.; Klumphu, P.; Lipshutz, B. H. Nat. Commun. 2015, 6, 7401.
(o) Cho, B. S.; Chung, Y. K. Chem. Commun. 2015, 51, 14543.
(p) Xiao, J.; Wen, H.; Wang, L.; Xu, L.; Hao, Z.; Shao, C.-L.; Wang, C.-Y. Green Chem. 2016, 18, 1032.
(q) Zhang, Y.; Wei, B.-W.; Lin, H.; Zhang, L.; Liu, J.-X.; Luo, H.-Q.; Fan, X.-L. Green Chem. 2015, 17, 3266.
(r) Ren, N.; Nie, J.; Ma, J.-A. Green Chem. 2016, 18, 6609.
[18] (a) Wang, J.; Kong, W.-G.; Li, F.; Liu, J.; Shen, Q.; Liu, L.; Zhao, W.-X. Org. Biomol. Chem. 2015, 13, 5399.
(b) Li, F.; Wang, J.; Xu, M.; Zhao, X.; Zhou, X.; Zhao, W.-X.; Liu, L. Org. Biomol. Chem. 2016, 14, 3981.
(c) Wang, J.; Li, F.; Shen, Q.; Pei, W.; Zhao, W.-X.; Liu, L. Synthesis 2016, 48, 441.
(d) Wang, J.; Li, F.; Xie, H.; Xu, M.; Zhao, X.; Liu, L.; Zhao, W.-X. Appl. Organomet. Chem. 2017, 31, e3545.
[19] CCDC 1558716(3a) and CCDC 1559048(5) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Center via www.ccdc.cam.ac.uk/data_request/cif.
[20] (a) Creary, X. J. Org. Chem. 1987, 52, 5026.
(b) Chong, J. M.; Mar, E. K. J. Org. Chem. 1991, 56, 893.
(c) Prakash, G. K. S.; Panja, C.; Vaghoo, H.; Surampudi, V.; Kultyshev, R.; Mandal, M.; Rasul, G.; Mathew, T.; Olah, G. A. J. Org. Chem. 2006, 71, 6806.
(d) Cheng, H.; Pei, Y.; Leng, F.; Li, J.; Liang, A.; Zou, D.; Wu, Y.; Wu, Y. Tetrahedron Lett. 2013, 54, 4483.
[21] (a) Adamo, M. F. A.; Duffy, E. F. Org. Lett. 2006, 8, 5157.
(b) Adamo, M. F. A.; Suresh, S. Tetrahedron 2009, 65, 990.
(c) Zhang, J.; Liu, X.; Ma, X.; Wang, R. Chem. Commun. 2013, 49, 9329.
[22] Gao, J.-R.; Wu, H.; Xiang, B.; Yu, W.-B.; Han, L.; Jia, Y.-X. J. Am. Chem. Soc. 2013, 135, 2983.
[23] Fiandra, C. D.; Piras, L.; Fini, F.; Disetti, P.; Moccia, M.; Adamo, M. F. A. Chem. Commun. 2012, 48, 3863.
[24] McBee, E. T.; Kim, Y. S.; Braendlin, H. P. J. Am. Chem. Soc. 1962, 84, 3154.
/
| 〈 |
|
〉 |