

Chinese Journal of Organic Chemistry >
Synthesis and Evaluation of Some New Aza-A-homocholesteryl-lactam Thiazole Derivatives as Anticancer Agents
Received date: 2017-10-08
Revised date: 2017-12-13
Online published: 2018-02-06
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21462009, 21562007), the Technology and Development Project of Nanning City (No. 20171125-5), the Guangxi Colleges and University Key Laboratory Foundation of Beibu Gulf Oil and Natural Gas Resource Effective Utilization (No. 2014KLOG09).
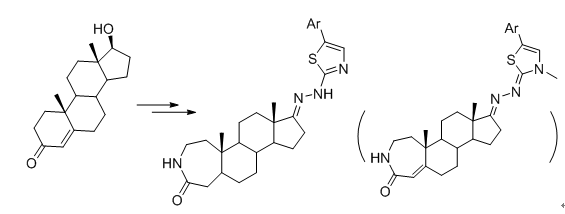
Using a testosterone as a raw material, a steroidal skelecton with A-homo-lactam was constructed. Then, some new aza-A-homocholesteryl-lactam thiazole derivatives were synthesized by modifying of the 17-hydroxyl and introducing of various heterocycles, and their structures were determined by IR, NMR and HRMS. The antiproliferative activity of the compounds against different carcinoma cells was assayed. The results show that some compounds display a distinct antiproliferative activity against tested cancer cells and are almost inactive to HEK293T cells.
Huang Yanmin , Pang Chunling , Yang Chunhui , Zhan Junyan , Gan Chunfang , Pang Liping , Liu Xiaolan , Cui Jianguo . Synthesis and Evaluation of Some New Aza-A-homocholesteryl-lactam Thiazole Derivatives as Anticancer Agents[J]. Chinese Journal of Organic Chemistry, 2018 , 38(6) : 1484 -1492 . DOI: 10.6023/cjoc201710005
[1] Festi, D.; Montagnani, M.; Azzaroli, F.; Lodato, F.; Mazzella, G.; Roda, A.; Di-Biase, A. R; Roda, E.; Simoni, P.; Colecchia, A. Curr.Clin.Pharmacol. 2007, 2, 155.
[2] Ifere, G.; Barr, E.; Equan, A.; Gordon, K.; Singh, U. P.; Chaudhary, J.; Igietseme, J. U.; Ananaba, G. A. Cancer Detect. Prev. 2009, 32, 319.
[3] Morzycki, J. W. Steroids 2014, 83, 62.
[4] Joao, F. S.; Carvalho, M.; Manuel, C. S.; Joao, N.; Moreira, S. S.; Melo, M. L. S. J.Med. Chem. 2011, 54, 6375.
[5] Jorge, A. R.; Salvador, J. F. S.; Carvalho, Marco A. C.; Neves, S. M.; Silvestre, A. J.; Leitao, M.; Manuel C. S. Nat. Prod.Rep. 2013, 30, 324.
[6] Ranju, B.; Pratap, C. A. Chem. Rev. 2014, 114, 6986.
[7] Liun, Q.-F.; Cui, J.-G.; Gan, C.-F. Chin. J.Org.Chem. 2012, 32, 2214(in Chinese). (林启福, 崔建国, 甘春芳, 刘亮, 姚秋翠, 黄燕敏, 有机化学, 2012, 32, 2214.)
[8] Huang, Y. M.; Cui, J. G.; Zhong, Z. G.; Gan, C. F.; Zhang, W. Y.; Song, H. C. Bioorg.Med.Chem.Lett. 2011, 21, 3641.
[9] Huang, Y. M.; Cui, J. G.; Chen, S. J.; Gan, C. F.; Zhou, A. M. Steroids 2011, 76, 1346.
[10] Huang, Y. M.; Cui, J. G.; Chen, S. J.; Lin, Q. F.; Song, H. C.; Gan, C.-F.; Su, B.; Zhou, A. M. Mar.Drugs 2014, 12, 1715.
[11] Huang, Y. M.; Cui, J. G.; Chen, S. J.; Gan, C.-F.; Yao, Q. C.; Lin, Q. F. Bioorg.Med.Chem.Lett. 2013, 23, 2265.
[12] Cui, S. F.; Wang, Y.; Lv, J. S.; Damu Guri L. V.; Zhou C. H. Sci. China Chem. 2012, 42, 1105(in Chinese). (崔胜峰, 王艳, 吕敬松, Damu Guri L. V., 周成合, 中国科学:化学, 2012, 42, 1105.)
[13] Kashfi, K. Adv. Pharmacol. 2009, 57, 31.
[14] Sun, L. P.; Jiang, Z.; Gao, L. X.; Liu, X. F.; Quan, Y. C.; Zheng, G. H.; Li, J.; Piao, H. Chin. J. Org. Chem. 2013, 33, 1496.
[15] Mohareb, R. M.; Al-Omran, F. Steroids 2012, 77, 1551.
[16] Cui, J. G.; Liu, L.; Gan, C. F.; Xiao, Q.; Huang, Y. M. Prog. Chem. 2014, 26, 320(in Chinese). (崔建国, 刘亮, 甘春芳, 肖琦, 黄燕敏, 化学进展, 2014, 26, 320.)
[17] Cui, J. G.; Zhao, D. D.; He, D. M.; Huang, Y. M.; Liu, Z. P.; Liu, X. F.; Shi, H. X.; Gan, C. F. Chin. J. Org. Chem. 2016, 36, 630(in Chinese). (崔建国, 赵丹丹, 何冬梅, 黄燕敏, 刘志平, 林啟福, 石海信, 甘春芳, 有机化学, 2016, 36, 630.)
[18] Huang, Y. M.; Qi, B. B.; Cui, J. G.; Gan, C. F.; Yang, C. H.; Liu, C.; Chen, S.; Shi, H. X. Chin. J. Org. Chem. 2016, 36, 1602(in Chinese). (黄燕敏, 戚斌斌, 崔建国, 甘春芳, 杨春晖, 刘畅, 陈爽, 石海信, 有机化学, 2016, 36, 1602.)
/
| 〈 |
|
〉 |