

Chinese Journal of Organic Chemistry >
Application of [4+2]Cycloaddition Reaction of Tetrazine with Cyclooctyne in the Construction of Pyridazine Structure with Axial Chirality
Received date: 2017-12-27
Revised date: 2018-01-29
Online published: 2018-02-11
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 91413107, 21432003), the Thousand Young Talents Program and the Fundamental Research Funds for the Central Universities of China (No. lzujbky-2014-61).
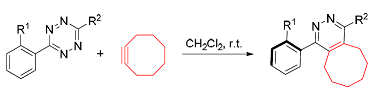
The application of [4+2] cycloaddition reaction of tetrazine with cyclooctyne in the construction of pyridazine structure with axial chirality was studied. The inverse electronic demand Diels-Alder reaction of tetrazine bearing bulky groups with macrocyclic tension’s cyclooctyne could take place under catalyst-free conditions in dichloromethane. The reaction underwent a six-membered bridged transition state, gently release a molecule of nitrogen to get axial chiral pyridazine structure. The transformation of the reaction can be determined by the change of color. The reaction could get potential axial chiral pyridazine structure with high yiled (95%) under mild conditions.
Cai Zhengjun , Gao Jianbao , Li Bai , Zhong Yuan , Feng Xing , Xue Jijun , Jiang Xianxing . Application of [4+2]Cycloaddition Reaction of Tetrazine with Cyclooctyne in the Construction of Pyridazine Structure with Axial Chirality[J]. Chinese Journal of Organic Chemistry, 2018 , 38(5) : 1138 -1146 . DOI: 10.6023/cjoc201712039
[1] Bringmann, G.; Gulder, T.; Gulder, T. A.; Breuning, M. Chem. Rev. 2010, 111, 563.
[2] Bringmann, G.; Price Mortimer, A. J.; Keller, P. A.; Gresser, M. J.; Garner, J.; Breuning, M. Angew. Chem., Int. Ed. 2005, 44, 5384.
[3] Christ, F.; Voet, A.; Marchand, A.; Nicolet, S.; Desimmie, B. A.; Marchand, D.; Bardiot, D.; Van der Veken, N. J.; Van Remoortel, B.; Strelkov, S. V. Nat. Chem. Biol. 2010, 6, 442.
[4] Kozlowski, M. C.; Morgan, B. J.; Linton, E. C. Chem. Soc. Rev. 2009, 38, 3193.
[5] Chen, Y.; Yekta, S.; Yudin, A. K. Chem. Rev. 2003, 103, 3155.
[6] Noyori, R.; Takaya, H. Acc. Chem. Res. 1990, 23, 345.
[7] Zamfir, A.; Schenker, S.; Freund, M.; Tsogoeva, S. B. Org. Biomol. Chem. 2010, 8, 5262.
[8] Cardoso, F. S.; Abboud, K. A.; Aponick, A. J. Am. Chem. Soc. 2013, 135, 14548.
[9] Fernández, E.; Guiry, P. J.; Connole, K. P.; Brown, J. M. J. Org. Chem. 2014, 79, 5391.
[10] Knöpfel, T. F.; Aschwanden, P.; Ichikawa, T.; Watanabe, T.; Carreira, E. M. Angew. Chem. 2004, 116, 6097.
[11] Milhau, L.; Guiry, P. J. Synlett 2011, 383.
[12] Ramírez-López, P.; Ros, A.; Romero-Arenas, A.; Iglesias-Sigüenza, J.; Fernández, R.; Lassaletta, J. M. J. Am. Chem. Soc. 2016, 138, 12053.
[13] Miyaji, R.; Asano, K.; Matsubara, S. J. Am. Chem. Soc. 2015, 137, 6766.
[14] Hornillos, V.; Ros, A.; Ramírez-López, P.; Iglesias-Sigüenza, J.; Fernández, R.; Lassaletta, J. M. Chem. Commun. 2016, 52, 14121.
[15] Ramirez-Lopez, P.; Ros, A.; Estepa, B.; Fernández, R.; Fiser, B.; Gómez-Bengoa, E.; Lassaletta, J. M. ACS. Catal. 2016, 6, 3955.
[16] Zhang, J.-W.; Xu, J.-H.; Cheng, D.-J.; Shi, C.; Liu, X.-Y.; Tan, B. Nat. Commun. 2016, 7, 10677.
[17] Zhang, L.; Zhang, J.; Ma, J.; Cheng, D.-J.; Tan, B. J. Am. Chem. Soc. 2017, 139, 1714.
[18] Fan, X.; Ge, Y.; Lin, F.; Yang, Y.; Zhang, G.; Ngai, W. S. C.; Lin, Z.; Zheng, S.; Wang, J.; Zhao, J. Angew. Chem., Int. Ed. 2016, 55, 14046.
[19] Karver, M. R.; Weissleder, R.; Hilderbrand, S. A. Bioconjugate Chem. 2011, 22, 2263.
[20] Wu, H.; Yang, J.; Šeckute, J.; Devaraj, N.K. Angew. Chem., Int. Ed. 2014, 53, 5805.
[21] Boger, D. L. Tetrahedron 1983, 39, 2869.
[22] Zheng, S.-C.; Wu, S.; Zhou, Q.; Chung, L. W.; Ye, L.; Tan, B. Nat. Commun. 2017, 8, 15238.
[23] Ahmed, A.; Bragg, R. A.; Clayden, J.; Lai, L. W.; McCarthy, C.; Pink, J. H.; Westlund, N.; Yasin, S. A. Tetrahedron 1998, 54, 13277.
[24] Rana, S.; Haque, R.; Santosh, G.; Maiti, D. Inorg. Chem. 2013, 52, 2927.
[25] Belaud-Rotureau, M.; Castanet, A.-S.; Nguyen, T. H.; Mortier, J. Aust. J. Chem. 2016, 69, 307.
[26] Henlin, J.; Duchêne-Roger, F.; Desmet-Beaufort, C.; Levens, N.; Fauchère, J.; Boutin, J.; Nicolas, J. Chem. Biol. Drug. Des. 2001, 57, 419.
[27] Wang, D.; Chen, W.; Zheng, Y.; Dai, C.; Wang, L.; Wang, B. Heterocycl. Commun. 2013, 19, 171.
[28] Garcia-Hartjes, J.; Dommerholt, J.; Wennekes, T.; van Delft, F. L.; Zuilhof, H. Eur. J. Org. Chem. 2013, 3712.
[29] Bloom, S.; Knippel, J. L.; Holl, M. G.; Barber, R.; Lectka, T. Tetrahedron 2014, 55, 4576.
[30] Chen, W.-X.; Wang, D.-Z.; Dai, C.-F.; Hamelberg, D.; Wang, B.-H. Chem. Commun. 2012, 48, 1736.
/
| 〈 |
|
〉 |