

Chinese Journal of Organic Chemistry >
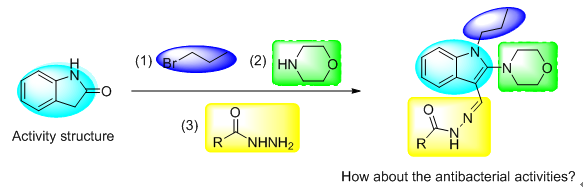
Synthesis and Antibacterial Activity of 2-Morpholino-1-propyl-1H-indole-3-substituted Acylhydrazone Derivatives
Received date: 2017-12-22
Revised date: 2018-02-06
Online published: 2018-02-28
Supported by
Project supported by the Guizhou Provincal Science Technology Program (No. 20161055), the Student Innovation and Entrepreneurship Project of Guizhou University (No. 201610657052), and the State Key Laboratory of Functions and Applications of Medicinal Plants (No. 201707).
A series of 2-morpholino-1-propyl-1H-indole-3-substituted acylhydrazone derivatives were designed and synthesized from oxindole. All the compounds were characterized by 1H NMR, 13C NMR and HRMS spectra, and their antibacterial activities were evaluated via turbidimeter test in vitro. The preliminary bioassay results indicated that all of the title compounds exhibited certain antibacterial activities in vitro against Xanthomonas axonopodis pv. Citri (X. citri), Ralstonia.solanacearum (R. solanacearum) and Xanthomonas oryzae pv. Oryzae (X. oryzae). These title compounds 2-cyano-N'-((2-morpholino-1-propyl-1H-indol-3-yl)methylene)acetohydrazide (12a), 4-chloro-N'-((2-morpholino-1-propyl-1H-indol-3-ylmethylene)ben-zohydrazide (12c), 4-fluoro-N'-((2-morpholino-1-propyl-1H-indol-3-yl)methylene)benzohydrazide (12f), N'-((2-morpholino-1-propyl-1H-indol-3-yl)methylene)-4-nitrobenzohydrazide (12k) and N'-((2-morpholino-1-propyl-1H-indol-3-yl)methylene) isonicotinohydrazide (12m) displayed good antibacterial activities. The concentration of 50% inhibition rate (EC50) of 12a, 12c, 12f, 12k and 12m against X. oryzae were 73.79, 61.94, 59.70, 36.72 and 82.79 μg/mL respectively, which was significantly superior to the control bismerthiazol and thiodiazole-copper (92.46 μg/mL and 120.22 μg/mL).
Key words: indole derivatives; acylhydrazone; acylhydrazine; antibacterial activity
Wu Shouqun , Li Xiaoqin , Meng Jiao , Gan Yiyuan , Tian Kun , Wang Zhenchao , Ouyang Guiping . Synthesis and Antibacterial Activity of 2-Morpholino-1-propyl-1H-indole-3-substituted Acylhydrazone Derivatives[J]. Chinese Journal of Organic Chemistry, 2018 , 38(6) : 1447 -1453 . DOI: 10.6023/cjoc201712030
[1] Agarwal, A.; Srivastava, K.; Puri, S. K.; Chauhan, P. M. S. Bioorg.Med.Chem.Lett. 2005, 15, 3133.
[2] Li, Q.; Wang, Y.; Hu, M. J.; Chen, P.; You, W. W.; Zhao, P. Chin.J.Org.Chem. 2017, 37, 967(in Chinese). (黎秋, 汪雨, 胡孟金, 陈鹏, 游文玮, 赵培亮, 有机化学, 2017, 37, 967.)
[3] Kaushik, N. K.; Kaushik, N.; Attri, P.; Kumar, N.; Kim, C. H.; Verma, A. K.; Choi, E. H. Molecules 2013, 18, 6620.
[4] Sharma, V.; Kumar, P.; Pathak, D. J.Heterocycl. Chem. 2010, 47, 491.
[5] Skibo, E. B.; Xing, C.; Dorr, R. T. J.Med. Chem. 2001, 44, 3545.
[6] Cai, M.; Hu, J.; Tian, J. L.; Yan, H.; Zheng, C. G.; Hu, W. L. Chin.Chem.Lett. 2015, 26, 675.
[7] Saundane, A. R.; Vijaykumar, K.; Yarlakatti, M.; Prabhaker, W.; Vaijinath, A. V. Heterocycl. Lett. 2011, 1, 339.
[8] Rindhe, S. S.; Karale, B. K.; Gupta, R. C.; Rode, M. A. Indian J Pharm Sci. 2011, 73, 292.
[9] Ma, H. L.; Yan, X. J.; Xiao, Y. M.; Yuan, D. K.; Zhang, Z. H.; Fu, B.; Yuan, H. Z. Chin.J.Org.Chem. 2016, 36, 158(in Chinese). (麻红利, 闫晓静, 肖玉梅, 袁德凯, 张振华, 傅滨, 袁会珠, 有机化学, 2016, 36, 158.)
[10] Tiwari, R. K.; Singh, D.; Singh, J.; Yadav, V.; Pathak, A. K.; Dabur, R.; Chhillar, A. K.; Singh, R.; Sharma, G. L.; Chandra, R.; Verma, A. K. Bioorg.Med.Chem.Lett. 2006, 16, 413.
[11] Huffman, J. W.; Zengin, G.; Wu, M. J.; Lu, J.; Hynd. G.; Bushell, K.; Thompson, A. S.; Bushell, S.; Tartal, C.; Hurst, D. P.; Reggio, P. H.; Selley, D. E.; Cassidy, M. P.; Wiley, J. L.; Martin, B. R. Bioorg.Med. Chem. 2005, 13, 89.
[12] Pandeya, S. N.; Sriram, D.; Nath, G.; DeClercq, E. Eur.J. Pharm.Sci. 1999, 9, 25.
[13] Tu, H.; Wu, S. Q.; Li, X. Q.; Wang, Z. C.; Wan, J. L.; Tian, K.; Ouyang, G. P. J.Heterocycl. Chem. 2017, 00, 00.
[14] Ravikumar, C.; Joe, I. H.; Jayakumar, V. S. Chem. Phys. Lett. 2008, 460, 552.
[15] Xiang, Y.; Tong, A. J.; Jin, P. Y; Ju, Y. Org. Lett. 2006, 8, 2863.
[16] Ozkay, Y.; Tunali, Y.; Karaca, H.; IsIkdag, I. Eur. J. Med. Chem. 2010, 45, 3293.
[17] Fan, z. j.; Zhong. B.; Wang, S. H.; Li, Z. M. Chin. J. Appl. Chem. 2003, 20, 365(in Chinese). (范志金, 钟滨, 王素华, 李正名, 应用化学, 2003, 20, 365.)
[18] Obulesu, O.; Nanubolu, J. B.; Suresh, S. Org.Biomol.Chem. 2015, 13, 8232.
[19] Gao, W. T.; Gao, D. P; Guo, H. Chem.Res.Chin. Univ. 2009, 25, 465.
[20] Singh, P.; Kumar, R.; Yadav, B.; Khanna, R. S.; Tewari, A. K. RSC Adv. 2014, 4, 51239.
[21] Munawar, M. A.; Chattha, F. A. K.; Kousar, S.; Munir, J.; Ismail, T.; Ashraf, M.; Khan, M. A. Bioorg.Med.Chem. 2015, 23, 6014.
[22] Palace-Berl, F.; Jorge, S. D.; Pasqualoto, K. F. M.; Ferreira, A. K.; Maria, D. A.; Zorzi, R. R.; Bortolozzo, L. D. S.; Lindoso, J. A. L.; Tavares, L. C. Bioorg.Med.Chem., 2013, 21, 5395.
[23] Khan, K. M.; Salar, U.; Fakhri, M. I.; Taha, M.; Hameed, A.; Perveen, S.; Voelter, W. Lett.Org.Chem. 2015, 12, 637.
[24] Kamal, A.; Srikanth, P. S.; Vishnuvardhan, M. V. P. S.; Kumar, G. B.; Babu, K. S.; Hussaini, S. A.; Alarifi, A. Bioorg.Chem. 2016, 65126.
[25] Backes, G. L.; Jursic, B. S.; Neumann, D. M. Bioorg. Med. Chem. 2015, 23, 3397.
/
| 〈 |
|
〉 |