

Chinese Journal of Organic Chemistry >
Amidation of Acid Chlorides to Primary Amides with Ammonium Salts
Received date: 2018-01-24
Revised date: 2018-03-06
Online published: 2018-03-08
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21672199, 21702197), the CAS Interdisciplinary Innovation Team, the Fundamental Research Funds for the Central Universities and the Anhui Provincial Natural Science Foundation (No. 1708085MB28).
A practical amidation reaction for the synthesis of primary amides is presented, in which the simple NH4Cl was identified as a practical and convenient amine source. A series of aromatic and aliphatic acid chlorides were successfully compatible with this protocol, affording the corresponding amides in good to excellent yields, which provides a rapid and reliable approach to amides from simple starting materials. Introducing the appropriate N-methyl pyrrolidone (NMP) into the system as solvent and acid-binding reagent plays a key role to avoid the use of stoichiometric amounts of base.
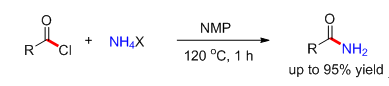
Key words: ammonium salts; amidation; primary amides; acid chlorides; N-methyl pyrrolidone
Li Zhejian , Gao Bao , Huang Hanmin . Amidation of Acid Chlorides to Primary Amides with Ammonium Salts[J]. Chinese Journal of Organic Chemistry, 2018 , 38(6) : 1431 -1436 . DOI: 10.6023/cjoc201801034
[1] (a) Constable, D. J. C.; Dunn, P. J.; Hayler, J. D.; Humphrey, G. R.; Leazer, J. L. Jr.; Linderman, R. J.; Lorenz, K.; Manley, J.; Pearlman, B. A.; Wells, A.; Zaks, A.; Zhang, T. Y. Green Chem. 2007, 9, 411.
(b) Montalbetti, C. A. G. N.; Falque, V. Tetrahedron 2005, 61, 10827.
(c) Ghose, A. K.; Viswanadhan, V. N.; Wendoloski, J. J. J. Comb. Chem. 1999, 1, 55.
[2] (a) Pattabiraman, V. R.; Bode, J. W. Nature 2011, 480, 471.
(b) Allen, C. L.; Williams, J. M. J. Chem. Soc. Rev. 2011, 40, 3405.
(c) Gunanathan, C.; Ben-David, Y.; Milstein, D. Science 2007, 317, 790.
(d) Zhu, J.; Gao, B.; Huang, H. Org. Biomol. Chem. 2017, 15, 2910.
(e) Yu, X.-Y.; Zhou, F.; Chen, J.-R.; Xiao, W.-J. Acta Chim. Sinica 2017, 75, 86(in Chinese). (余晓叶, 周帆, 陈加荣, 肖文精, 化学学报, 2017, 75, 86.)
[3] (a) Hirano, T.; Uehara, K.; Kamata, K.; Mizuno, N. J. Am. Chem. Soc. 2012, 134, 6425.
(b) Zhang, S.; Xu, H.; Lou, C.; Senan, A. M.; Chen, Z.; Yin, G. Eur. J. Org. Chem. 2017, 2017,1870.
(c) Veisi, H.; Maleki, B.; Hamelian, M.; Ashrafi, S. S. RSC Adv. 2015, 5, 6365.
(d) Marce, P.; Lynch, J.; Blacker, A. J.; Williams, J. M. J. Chem. Commun. 2016, 52, 1436.
(e) Ramon, R. S.; Marion, N.; Nolan, S. P. Chem.-Eur. J. 2009, 15, 8695.
[4] (a) Jagdmann, G. E. Jr.; Munson, H. R. Jr.; Gero, T. W. Synth. Commun. 1990, 20, 1203.
(b) Griffin, J.; Atherton, J.; Page, M. I. J. Phys. Org. Chem. 2013, 26, 1032.
(c) Arrizabalaga, P.; Castan P.; Laurent, J.-P. J. Am. Chem. Soc. 1984, 106, 4814.
(d) Garcia, J.; Gonzadez, J.; Segura, R.; Urpi, F.; Vilarrasa, J. J. Org. Chem. 1984, 49, 3322.
[5] Khalafi-Nezhad, A.; Zare, A.; Parhami, A.; Rad, M. N. S.; Nejabat, G. R. Phosphorus, Sulfur Silicon Relat. Elem. 2007, 182, 657.
[6] (a) Cui, Z.; Ling, Y.; Li, B.; Li, Y.; Rui, C.; Cui, J.; Shi, Y.; Yang, X. Molecules 2010, 15, 4267.
(b) Wang, L.; Dai, F.-Y.; Zhu, J.; Dong, K.-K.; Wang, Y.-L.; Chen, T. J. Chem. Res. 2011, 35, 313.
(c) Srinivasan, S.; Manisankar, P. Synth. Commun. 2010, 40, 3538.
[7] (a) Kent, R. E.; McElvain, S. M. Org. Synth. 1955, 3, 490.
(b) Fisher, L. E.; Caroon, J. M.; Stabler, S. R.; Lundberg, S.; Zaidi, S.; Sorensen, C. M.; Sparacino, M. L.; Muchowski, J. M. Can. J. Chem. 1994, 72, 142.
(c) Keurulainen, L.; Heiskari, M.; Nenonen, S.; Nasereddin, A.; Kopelyanskiy, D.; Leino, T. O.; Yli-Kauhaluoma, J.; Jaffe, C. L.; Kiuru, P. Med. Chem. Commun. 2015, 6, 1673.
(d) Farcasiu, D.; Jahme, J.; Rüchardt, C. J. Am. Chem. Soc. 1985, 107, 5717.
[8] (a) Green, R. A.; Hartwig, J. F. Org. Lett. 2014, 16, 4388.
(b) Kim, J.; Chang, S. Chem. Commun. 2008, 3052.
(c) Suresh, A. S.; Baburajan, P.; Ahmed, M. Tetrahedron Lett. 2015, 56, 4864.
(d) Wu, X.; Wannberg, J.; Larhed, M. Tetrahedron 2006, 62, 4665.
[9] (a) Della, E. W.; Kasum, B.; Kirkbride, K. P. J. Am. Chem. Soc. 1987, 109, 2746.
(b) Ma, Y.; Stivala, C. E.; Wright, A. M.; Hayton, T.; Liang, J.; Kersztes, I.; Lobkovsky, E.; Collum, D. B.; Zakarian, A. J. Am. Chem. Soc. 2013, 135, 16853.
(c) Sakharov, S. G.; Kovalev, V. V.; Gorbunova, Y. E.; Tokmakov, G. P.; Skabitskii, I. V.; Kokunov, Y. V. Russ. J. Coord. Chem. 2017, 43, 75.
(d) Caner, J.; Vilarrasa, J. J. Org. Chem. 2010, 75, 4880.
[10] (a) Gao, B.; Zhang, G.; Zhou, X.; Huang, H. Chem. Sci. 2018, 9, 380.
(b) Zhang, G.; Ji, X.; Yu, H.; Yang, L.; Jiao, P.; Huang, H. Tetrahedron Lett. 2016, 57, 383.
(c) Hu, Y.; Shen, Z.; Huang, H. ACS Catal. 2016, 6, 6785.
(d) Zhang, G.; Gao, B.; Huang, H. Angew. Chem., Int. Ed. 2015, 54, 7657.
[11] Liu, J.; Li, H.; Spannenberg, A.; Franke, R.; Jackstell, R.; Beller, M. Angew. Chem., Int. Ed. 2016, 55, 13544.
[12] (a) Yamada, K.; Karuo, Y.; Tsukada, Y.; Kunishima, M. Chem.-Eur. J. 2016, 22, 14042.
(b) Liu, H.; Laurenczy, G.; Yan, N.; Dyson, P. J. Chem. Commun. 2014, 50, 341.
(c) Song, Q.; Feng, Q.; Yang, K. Org. Lett. 2014, 16, 624.
(d) Ma, X.-Y.; He, Y.; Hu, Y.-L.; Lu, M. Tetrahedron Lett. 2012, 53, 449.
(e) Freudenreich, C.; Samama, J.-P.; Biellmann, J.-F. J. Am. Chem. Soc. 1984, 106, 3344.
(f) Khrustalev, V. N.; Sandhu, B.; Bentum, S.; Fonari, A.; Krivoshein, A. V.; Timofeeva, T. V. Cryst. Growth Des. 2014, 14, 3360.
(g) Blanchette, J. A.; Brown, E. V. J. Am. Chem. Soc. 1952, 74, 1066.
(h) Movassagh, B.; Rezaei, N. New J. Chem. 2015, 39, 7988.
(i) Mol, M. D. Recl. Trav. Chim. Pays-Bas 1907, 26, 373.
(j) Martinez-Asencio, A.; Yus, M.; Ramon, D. J. Tetrahedron 2012, 68, 3948.
/
| 〈 |
|
〉 |