

Chinese Journal of Organic Chemistry >
Efficient and Chemoselective Deprotection of N-t-Butyloxycarbonyl Group Mediated by Selectfluor
Received date: 2018-01-25
Revised date: 2018-02-12
Online published: 2018-03-16
Supported by
Project supported by the National Natural Science Foundation of China (No. 21502076), the Natural Science Foundation of Jiangxi Province (No. 20161BAB213068), the Hundred-Talent Program (Hefei) and the Outstanding Young Talent Program of Jiangxi Province (No. 20171BCB23039).
Selectfluor, 1-chloromethyl-4-fluoro-1,4-diazoniabicyclo-[2.2.2] octane bis(tetrafluoroborate), is among the most popular fluorinating reagents owning to its commercially availability and non-hygroscopic property. The discovery and understanding of new reactivities of selectfluor are thus important for reaction design and optimization when this popular reagent is employed. It has been found that selectfluor could selectively remove Boc group from doubly protected amines in acetonitrile. This deprotection could be of interest when compared to other reported methods, not only because selectfluor is a solid and easy-to-handle, but also because the reaction is mild, operationally simple and chemoselective. The potential usefulness of this method is demonstrated by the deprotection of a series of protected amino acids and a one-step synthesis of pharmaceutically important purine derivative. The NMR experiments conducted in CD3CN explain why stoichiometric amount of selectfluor is needed for a successful reaction.
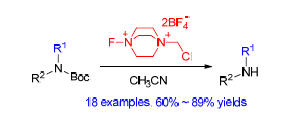
Key words: selectfluor; t-butyloxycarbonyl; deprotection; selectivity; synthesis
Zeng Yijie , Duan Yue , Zhao Hui , Hu Xiangguo . Efficient and Chemoselective Deprotection of N-t-Butyloxycarbonyl Group Mediated by Selectfluor[J]. Chinese Journal of Organic Chemistry, 2018 , 38(7) : 1712 -1717 . DOI: 10.6023/cjoc201801036
[1] (a) Banks, R. E.; Mohialdinkhaffaf, S. N.; Lal, G. S.; Sharif, I.; Syvret, R. G. J. Chem. Soc., Chem. Commun. 1992, 595.
(b) Nyffeler, P. T.; Duron, S. G.; Burkart, M. D.; Vincent, S. P.; Wong, C. H. Angew. Chem., Int. Ed. 2005, 44, 192.
[2] (a) Rueda-Becerril, M.; Sazepin, C. C.; Leung, J. C. T.; Okbinoglu, T.; Kennepohl, P.; Paquin, J. F.; Sammis, G. M. J. Am. Chem. Soc. 2012, 134, 4026.
(b) Yin, F.; Wang, Z. T.; Li, Z. D.; Li, C. Z. J. Am. Chem. Soc. 2012, 134, 10401.
(c) Yan, H.; Zhu, C. Sci. China:Chem. 2017, 60, 214.
[3] Stavber, S. Molecules 2011, 16, 6432.
[4] Greene, T. W.; Wuts, P. G. M. in Protective Groups in Organic Synthesis, Wiley, New York, 1999, pp. 518~525.
[5] Deniau, G.; Slawin, A. M. Z.; Lebl, T.; Chorki, F.; Issberner, J. P.; van Mourik, T.; Heygate, J. M.; Lambert, J. J.; Etherington, L. A.; Sillar, K. T.; O'Hagan, D. Chem. Biochem. 2007, 8, 2265.
[6] (a) Yadav, J. S.; Subba Reddy, B. V.; Reddy, K. S. Synlett 2002, 468.
(b) Mohapatra, D. K.; Durugkar, K. A. ARKIVOC 2005, 14, 20.
(c) Hernadez, J. N.; Crisostomo, F. R. P.; Martin, T.; Martin, V. S. Eur. J. Org. Chem. 2007, 5050.
(d) Zheng, J. L.; Yin, B. L.; Huang, W. M.; Li, X. P.; Yao, H. Q.; Liu, Z. G.; Zhang, J. C.; Jiang, S. Tetrahedron Lett. 2009, 50, 5094.
(e) Lopez-Soria, J. M.; Perez, S. J.; Hernandez, J. N.; Ramirez, M. A.; Martin, V. S.; Padron, J. I. RSC. Adv. 2015, 5, 6647.
(f) Walters, M. A.; Hoem, A. B. J. Org. Chem. 1994, 59, 2645.
(g) Hernández, J. N.; Ramírez, M. A.; Martín, V. S. J. Org. Chem. 2003, 68, 743.
(h) Boyle, T. P.; Bremner, J. B.; Coates, J. A.; Keller, P. A.; Pyne, S. G. Tetrahedron 2005, 61, 7271.
[7] (a) Hu, X. G.; Thomas, D. S.; Griffith, R.; Hunter, L. Angew. Chem., Int. Ed. 2014, 53, 6176.
(b) Hu, X. G.; Lawer, A.; Peterson, M. B.; Iranmanesh, H.; Ball, G. E.; Hunter, L. Org. Lett. 2016, 18, 662.
(c) Yan, N.; Fang, Z.; Liu, Q. Q.; Guo, X. H.; Hu, X. G. Org. Biomol. Chem. 2016, 14, 3469.
(d) Yan, N.; Lei, Z. W.; Su, J. K.; Liao, W. L.; Hu, X. G. Chin. Chem. Lett. 2017, 28, 467.
[8] Xia, J. B.; Zhu, C.; Chen, C. J. Am. Chem. Soc. 2013, 135, 17494.
[9] Comparison of specific rotations was used in Ref.
[11] for the same purpose.
[10] Dey, S.; Garner, P. J. Org. Chem. 2000, 65, 7697.
[11] (a) Liu, J. J.; Wong, C. H. Tetrahedron Lett. 2002, 43, 4037.
(b) Shah, S. T. A.; Singh, S.; Guiry, P. J. J. Org. Chem. 2009, 74, 2179.
[12] Navarre, L.; Martinez, R.; Genet, J. P.; Darses, S. J. Am. Chem. Soc. 2008, 130, 6159.
[13] Barrett, A. G. M.; Pilipauskas, D. J. Org. Chem. 1990, 55, 5170.
[14] Adams, H.; Bawa, R. A.; Jones, S. Org. Biomol. Chem. 2006, 4, 4206.
[15] Pandey, R. K.; Dagade, S. P.; Dongare, M. K.; Kumar, P. Synth. Commun. 2003, 33, 4019.
[16] Heller, S. T.; Sarpong, R. Org. Lett. 2010, 12, 4572.
[17] Wakasugi, K.; Iida, A.; Misaki, T.; Nishii, Y.; Tanabe, Y. Adv. Synth. Catal. 2003, 345, 1209.
[18] Mandal, P. K.; McMurray, J. S. J. Org. Chem. 2007, 72, 6599.
[19] Burk, M. J.; Allen, J. G. J. Org. Chem. 1997, 62, 7054.
[20] Zheng, J.; Yin, B.; Huang, W.; Li, X.; Yao, H.; Liu, Z.; Zhang, J.; Jiang, S. Tetrahedron Lett. 2009, 50, 5094.
[21] Tomita, K.; Oishi, S.; Ohno, H.; Fujii, N. Pept. Sci. 2008, 90, 503.
/
| 〈 |
|
〉 |