

Chinese Journal of Organic Chemistry >
Two New Sesquterpenoids from Fructus Carotae
Received date: 2018-01-19
Revised date: 2018-02-26
Online published: 2018-04-04
Supported by
Project supported by the National Natural Science Foundation of China (No. 31560102).
Seven sesquterpenoids were obtained from the Chinese traditional medicine, Fructus carotae, and their structures were identified as 7-ethoxy-4(15)-oppositen-1β-ol (1), 11-acetoxy-8β-angeloyloxy-15-methoxy-4α,5α-epoxyarbutane-3-one (2), 11-acetoxy-8β-propionyl-4-guaien-3-one (3), 1-oxo-5α,7αH-eudesma-3-en-15-al (4), 1β-hydroxy-4(15),7-eudesmadiene (5), 1β-hydroxy-4(15),5E,10(14)-germacratriene (6) and 1β-hydroxy-4(15),5-eudesmadiene (7). Compounds 1 and 2 were new sesquterpenoid compounds, and their structures were determined by the analysis of HR-ESIMS, 1D NMR and 2D NMR.
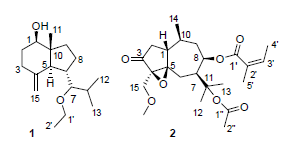
Key words: apiaceae; fructus carotae; arbutane; eudesmane; sesquiterpenoids
Cheng Lei , Liu Guiyuan , Pan Yinchi , Zhang Maosheng , Xiao Shiji . Two New Sesquterpenoids from Fructus Carotae[J]. Chinese Journal of Organic Chemistry, 2018 , 38(7) : 1829 -1832 . DOI: 10.6023/cjoc201801027
[1] Chinese Flora Editorial Board of Chinese Academy of Sciences Flora of China, Vol. 55, Science Press, Beijing, 1992, p. 223 (in Chinese).
(中国科学院中国植物志编辑委员, 中国植物志, 第55卷, 科学出版社, 北京, 1992, p. 223.)
[2] Zhang, H.; Gao, J. H.; Meng, L. J. Tianjin Med. Univ. 2004, 10, 492 (in Chinese).
(张卉, 高建华, 孟林, 天津医科大学学报, 2004, 10, 492.)
[3] Yang, R. L.; Yan, Z. H.; Lu, Y. J. Agric. Food Chem. 2008, 56, 3024.
[4] Pant, B.; Manandhar, S. Tumori 2010, 5, 461.
[5] Tavares, A. C.; Gonçalves, M. J.; Cavaleiro, C. J. Ethnopharmacol. 2008, 19, 129.
[6] Gebhardt, Y.; Witte, S.; Forkmann, G. Phytochemistry 2005, 66, 1273.
[7] Fu, H. W.; Zhang, L.; Yi, T.; Feng, Y. L.; Tian, J. K. Biochem. Syst. Ecol. 2010, 38, 309.
[8] Fu, Z. Z.; Han, H. T.; Liu, N.; Xu, X. B.; Zhu, W.; Gong, M. H.; Zhang, L.; Tian, J. K. Phytochem. Lett. 2015, 14, 35.
[9] Xiao, S. J.; Shi, D. B.; Yuan, Z. L.; Chen, Y. Z.; Zhang, M. S.; Ding, L. S.; Zhou, Y. Chin. J. Org. Chem. 2016, 36, 1686 (in Chinese).
(肖世基, 史大斌, 袁泽利, 陈永正, 张茂生, 有机化学, 2016, 36, 1686.)
[10] Liu, G. Y.; Wen, N.; Zhang, M. S.; Xu, Y. S.; Fu, S. B.; Xiao, S. J. Acta Pharm. Sinica 2017, 52, 1146 (in Chinese).
(刘贵园, 温楠, 张茂生, 徐应淑, 付少彬, 肖世基, 药学学报, 2017, 52, 1146.)
[11] Lee, I. K.; Lee, J. H.; Hwang, E. I.; Yun, B. S. Chem. Pharm. Bull. 2008, 56, 1483.
[12] Gao, X.; Deng, X. H. J. Chem. Res. 2009, 7, 457.
[13] Sun, Z. H.; Chen, B.; Zhang, S.; Hu, C. Q. J. Nat. Prod. 2004, 67, 1975.
[14] Brown, G. D.; Liang, G. Y.; Sy, L. K. Phytochemistry 2003, 64, 303.
[15] Xiao, S. J.; Chen, F.; Ding, L. S.; Zhou, Y. Chin. J. Nat. Med. 2015, 13, 65.
[16] Yang, M. C.; Lee, K. H.; Kim, K. H.; Choi, S. U.; Lee, K. R. Arch. Pharmacal. Res. 2007, 30, 1067.
[17] Kwak, Y. G.; Kim, D. K.; Ma, T. Z.; Park, S. A.; Park, H.; Jung, Y. H.; Yoo, D. J.; Eun, J. S. Arch. Pharmacal Res. 2006, 29, 834.
/
| 〈 |
|
〉 |