

Chinese Journal of Organic Chemistry >
Synthesis and Biological Evaluation of Piperazine Substituted 3-Aryl-5-furanyldihydropyrazole Amide Derivatives
Received date: 2018-02-06
Revised date: 2018-03-27
Online published: 2018-04-27
Supported by
Project supported by the National Natural Science Foundation of China (No. 81460624) and the Yunnan Provincial Science and Technology Department-Applied Basic Research Joint Special Funds of Yunnan University of Traditional Chinese Medicine (No. 2017FF117(-023)).
Pyrazole is a five-membered heterocyclic molecule with a broad range of biological activities. In this study, a series of new 3-aryl-5-furanyl-4,5-dihydropyrazole derivatives have been designed and synthesized by the general principle of molecular hybridization. The structures were characterized by 1H NMR, 13C NMR and HRMS. We screened in vitro anti-inflammatory in lipopolysaccharide (LPS)-stimulated RAW-264.7 macrophages and anticancer activity against 3 strains human tumor cell lines (A549, Hela and SGC7901) by the methyl thiazolyl tetrazolium (MTT) assay. The result indicated that dihydropyrazole compounds showed good inhibitory effect on the generation of NO and selective cytotoxic activity against tumor cell lines, and the acyl moieties of amides had an obvious influence on biological activities. Especially, 3 compounds were found to be similar anti-inflammatory to positive control dexamethasone, and 3 compounds displayed similar selective anti-tumor activity to positive control 5-fluorouracil (5-FU), which were to be lead compounds for further SAR research.
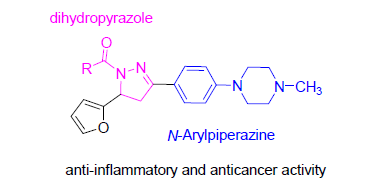
Key words: dihydropyrazole; amide; anti-inflammatory activity; anticancer activity
Mao Zewei , Liu Bei , Zhu Ping , Zhang Lijun , Zhu Jiahong , Wu Linze , Wan Chunping . Synthesis and Biological Evaluation of Piperazine Substituted 3-Aryl-5-furanyldihydropyrazole Amide Derivatives[J]. Chinese Journal of Organic Chemistry, 2018 , 38(8) : 2167 -2173 . DOI: 10.6023/cjoc201802010
[1] Chimenti, F.; Bizzarri, B.; Manna, F.; Bolasco, A.; Secci, D.; Chimenti, P.; Granese, A.; Rivanera, D.; Lilli, D.; Scaltrito, M. M.; Brenciaglia, M. I. Bioorg. Med. Chem. Lett. 2005, 15, 603.
[2] Chimenti, F.; Fioravanti, R.; Bolasco, A.; Manna, F.; Chimenti, P.; Secci, D.; Rossi, F.; Turini, P.; Ortus, F.; Alcaro, S.; Cardia, M. C. Eur. J. Med. Chem. 2008, 43, 2262.
[3] Zhang, Y. P.; Tan, W.; Yang, Y. S.; Sun, S. Q.; Guo, H. S. Chin. J. Org. Chem. 2015, 35, 1985(in Chinese). (张应鹏, 谭伟, 杨云裳, 孙世琪, 郭慧琛, 有机化学, 2015, 35, 1985.)
[4] Chundawat, T. S.; Sharma, N.; Bhagat, S. Med. Chem. Res. 2014, 23, 1350.
[5] Banday, A. H.; Shameem, S. A.; Jeelani, S. Steroids 2014, 92, 13.
[6] Deng, H.; Yu, Z. Y.; Shi, G. Y.; Chen, M. J.; Tao, K.; Hou, T. P. Chem. Biol. Drug Des. 2012, 79, 279.
[7] Ahmad, P.; Woo, H.; Jun, K. Y.; Kadi, A. A.; Abdel-Aziz, H. A.; Kwon, Y.; Rahman, A. F. M. M.. Bioorg. Med. Chem. 2016, 24, 1898.
[8] Amir, M.; Ali, S.; Somakala, K. Indian J. Chem. 2016, 55B, 478.
[9] Cetin, A.; Cansiz, A.; Digrak, M. Heteroat. Chem. 2003, 14, 345.
[10] Manna, K.; Agrawal, Y. K. Eur. J. Med. Chem. 2010, 45, 3831.
[11] Ma, Y. L.; Zheng, X.; Gao, H.; Wan, C. P.; Rao, G. X.; Mao, Z. W. Molecules 2016, 21, 1684.
[12] Mao, Z. W.; Zheng, X.; Qi, Y.; Zhang, M. D.; Huang, Y.; Wan, C. P.; Rao, G. X. RSC Adv. 2016, 6, 7723.
[13] Lin, Y. P.; Hu, C. Y.; Zheng, X.; Wang, X. L.; Wan, C. P.; Mao, Z. W. Chin. J. Org. Chem. 2017, 37, 237(in Chinese). (林玉萍, 虎春艳, 郑喜, 王秀丽, 万春平, 毛泽伟, 有机化学, 2017, 37, 237.)
[14] Li, Y. K.; Liu, B.; Zhang, L. J.; Zhu, J. H.; Wang, C. P.; Mao, Z. W. Chin. J. Org. Chem. 2018, 38, 949(in Chinese). (黎勇坤, 刘蓓, 张丽君, 朱加洪, 万春平, 毛泽伟, 有机化学, 2018, 38, 949.)
/
| 〈 |
|
〉 |