

Chinese Journal of Organic Chemistry >
Synthesis and Biological Activities of Novel 1,2,4-Triazole Triazene Derivatives
Received date: 2018-02-07
Revised date: 2018-03-31
Online published: 2018-05-17
Supported by
Project supported by the National Natural Science Foundation of China (No. J1210060), the Science and Technology Planning Project of Henan Province (No. 0624420031) and the Basic Research Development Program of Henan Province (No. 022463001).
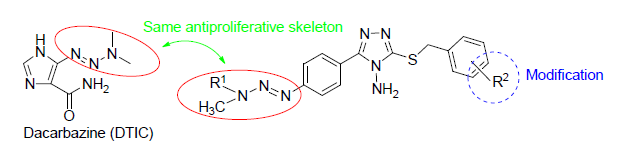
Sixteen novel 1,2,4-triazole triazene derivatives were synthesized in multiple steps from the p-aminobenzoic acid. Their structures were confirmed by 1H NMR, 13C NMR and HRMS. All the target compounds were evaluated for their anticancer activity. Among them, 2-[4-(3,3-dimethyltriazol-1-yl)phenyl]-3-amino-4-S-(4-chlorobenzyl)-1,2,4-triazole (6g), 2-[4-(3,3-dimethyltriazol-1-yl)phenyl]-3-amino-4-S-(2,4-dichlorobenzyl)-1,2,4-triazole (6h), 2-[4-(3,3-methylbenzyltriazen-1-yl)-phenyl]-3-amino-4-S-benzyl-1,2,4-trioxazole (6i), 2-[4-(3,3-methylbenzyltriazen-1-yl)phenyl]-3-amino-4-S-(4-methylbenzyl)-1,2,4-triazole (6j), 2-[4-(3,3-methylbenzyltriazen-1-yl)phenyl]-3-amino-4-S-(4-methoxybenzyl)-1,2,4-triazole (6l), 2-[4-(3,3-methylbenzyltriazen-1-yl)phenyl]-3-amino-4-S-(2,4-di-chlorobenzyl)-1,2,4-triazole (6p) exhibited good anti-cancer activity against J82 cells with the IC50 values of 23.883, 5.512, 8.731, 8.077, 5.590 and 12.195 μmol/L, respectively. And compounds 6h, 6i, 6j, 6l exhibited good anti-cancer activity against DU145 cells with the IC50 values of 13.690, 21.908, 10.772 and 4.827 μmol/L, respectively.
Key words: triazene; 1,2,4-triazole; synthesis; antitumor
Wei Guangpu , Zhang Xi , Lei Qiang , Xu Miao , Zhang Mingqian , Long Yue . Synthesis and Biological Activities of Novel 1,2,4-Triazole Triazene Derivatives[J]. Chinese Journal of Organic Chemistry, 2018 , 38(8) : 2137 -2142 . DOI: 10.6023/cjoc201802013
[1] Kumar, D.; Narayanam, M. K.; Chang, K. H.; Shah, K. Chem. Biol. Drug Des. 2011, 77, 182.
[2] Sumangala, V.; Poojary, B.; Chidananda, N.; Arulmoli, T.; Shenoy, S. Eur. J. Med. Chem. 2012, 54, 59.
[3] Al-Omar, M. A.; Al-Abdullah, E. S.; Shehata, I. A.; Habib, E. E.; Ibrahim, T. M.; El-Emam, A. A. Molecules 2010, 15, 2526.
[4] Abdel-Wahab, B. F.; Abdel-Latif, E.; Mohamed, H. A.; Awad, G. E. A. Eur. J. Med. Chem. 2012, 52, 263.
[5] Li, Y. C.; Cheng, J. R.; Zheng, J. Y. Chin. J. Pestic. 1993, 32, 11(in Chinese). (李煜昶, 成俊然, 郑健禺, 农药, 1993, 32, 11.)
[6] Li, Y. C. Chin. J. Pestic. 1993, 32, 20(in Chinese). (李煜昶, 农药, 1993, 32, 20.)
[7] Haria, M.; Bryson, H. M.; Goa, K. L. Drugs 1996, 50, 658.
[8] Casali, A.; Sega, F. M.; Casali, M.; Giuntini, T.; Cappellini, G. C.; Terzoli, E. J. Exp. Clin. Cancer Res. 2000, 19, 17.
[9] Goa, K. L.; Barradell, L. B. Drugs 1996, 51, 585.
[10] Stefan, B.; Stefan, D.; Frank, L.;Nicholas, E. L.; Emma, L. S. Bioorg. Med. Chem. Lett. 2002, 12, 1849.
[11] Koji, T.; Mohammad, A. A.; Naoyuki, H.; Johan, W.; Hiroshi, S.; Yoshitsugu, S.; Hayato, F.; Satoshi, S.; Mitsuhiro, A. J. Org. Chem. 2014, 79, 6366.
[12] Zhang, X. H.; Chen, W. B. Asian J. Chem. 2013, 25, 9805.
[13] Chu, J.; Xie, X.-H.; Yang, S. R.; Zhan, S. Z. Inorg. Chim. Acta 2014, 410, 191.
[14] Zhu, C. S.; Chen, W. B. Asian J. Chem. 2013, 25, 7409.
[15] Sun, H.; Wang, C. M.; Yang, Y. F.; Chen. P.; Wu, Y. D.; Zhang, X. H.; Huang, Y. J. Org. Chem. 2014, 79, 11863.
[16] Monteiro, A. S.; Almeida, J.; Cabral, G.; Severino, P.; Videira, P. A.; Sousa, A.; Nunes, R.; Pereira, J. D.; Francisco, A. P.; Perry, M. J.; Mendes, E. Eur. J. Med. Chem. 2013, 70, 1.
[17] Cappoen, D.; Vajs, J.; Uythethofken, C.; Kosmrlj, J. Eur. J. Med. Chem. 2014, 77, 193.
[18] Rodenko, B.; Wanner, M. J.; Alkhaldi, A. A. M.; Ebiloma, G. U.; Barnes, R. L.; Kaiser, M.; Brun, R.; Mccculloch, R.; Koomen, G. J.; Koning, H. P. Antimicrob. Agents Chemother. 2015, 59, 708.
[19] Lei, Q.; Zhang, S. Y.; Liu, M. L.; Li, J.; Zhang, X.; Long, Y. Mol. Diversity 2017, 21, 957.
[20] Lei, Q.; Qin, S. S.; Feng, C. N.; Li, P. P.; Zhang, X.; Long, Y. Chin. J. Org. Chem. 2016, 36, 406(in Chinese). (雷强, 秦上尚, 冯翠宁, 李佩佩, 张茜, 龙跃, 有机化学, 2016, 36, 406.)
[21] Yang, S. H. Chem. Intermed. 2008, (4), 20(in Chinese). (杨双花, 化工中间体, 2008, (4), 20.)
/
| 〈 |
|
〉 |