

Chinese Journal of Organic Chemistry >
Synthesis of 1,3-Diaryl or Dialkyl-2H-isoindole-4,7-dione via Barbier-Grignard-Type Reaction
Received date: 2018-04-04
Revised date: 2018-05-02
Online published: 2018-05-17
Supported by
Project supported by the National Natural Science Foundation of China (No. 21274083) and the National University Student Innovation and Entrepreneurship Training Program of China (No. 82617055).
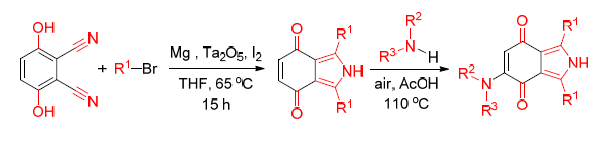
A novel, convenient and efficient protocol to N-heterocyclic derivatives of 1,3-diaryl or diaklyl-2H-isoin-dole-4,7-dione has been developed via the Barbier-Grignard-type reaction. Good to excellent yields up to 96% have been achieved by performing the reaction in one pot using Ta2O5 as the catalyst. The protocol displays good tolerance to different aryl and alkyl bromides. Based on some experimental results, a plausible mechanism involving nucleophilic addition and electron transfer has been proposed. The quinone structure in 1,3-diphenyl-2H-isoindole-4,7-dione (3a) makes it coupling with amine to form more complicated structure.
Key words: isoindole; Barbier-Grignard reaction; Ta2O5; oxidative coupling
Liu Hong , Dong Huiru , An Rui , Tang Yu , Xu Kaitian , Zhang Yuanming . Synthesis of 1,3-Diaryl or Dialkyl-2H-isoindole-4,7-dione via Barbier-Grignard-Type Reaction[J]. Chinese Journal of Organic Chemistry, 2018 , 38(8) : 2008 -2016 . DOI: 10.6023/cjoc201804007
[1] Gilman, H. Grignard Reactions of Nonmetallic Substances, Constable and Company, Ltd., London, 1954, p. 392.
[2] Stowell, J. C. Carbanions in Organic Synthesis, Wiley Inter-science, New York, 1979.
[3] Negishi, E. Organometallics in Organic Synthesis. Chemistry International——Newsmagazine for IUPAC 2006, 28, 27.
[4] Wakefield, B. J. Organomagnesium Methods in Organic Chemistry, Academic Press, New York, 1995.
[5] Wen, Y.; Chen, G.; Huang, S.; Tang, Y.; Yang, J.; Zhang, Y. Adv. Synth. Catal. 2016, 358, 947.
[6] Li, C. J.; Meng, Y. J. Am. Chem. Soc. 2000, 122, 9538.
[7] Shen, Z. L.; Yeo, Y. L.; Loh, T. P. J. Org. Chem. 2008, 73, 3922.
[8] Sormunen, G. J.; Lewis, D. E. Synth. Commun. 2004, 34, 3473.
[9] Pace, V.; Holzer, W.; Olofsson, B. Adv. Synth. Catal. 2014, 356, 3697.
[10] Wen, Y.; Chen, G.; Tang, Y.; Chen, J.; Yang, J.; Zhang, Y. Chin. J. Org. Chem. 2015, 35, 2545(in Chinese). (温运明, 陈桂芳, 唐渝, 陈静, 杨骏, 张渊明, 有机化学, 2015, 35, 2545.)
[11] Gao, F.; Deng, X.-J.; Tang, Y.; Tang, J.-P.; Yang, J.; Zhang, Y.-M. Tetrahedron Lett. 2014, 55, 880.
[12] Zhang, J.; Li, L. J.; Tang, Y.; Yang, J.; Xu, K. T.; Zhang, Y. M. ChemistrySelect 2017, 2, 5877.
[13] Chen, G.; Liu, H.; Li, S.; Tang, Y.; Lu, P.; Xu, K.; Zhang, Y. Org. Lett. 2017, 19, 1792.
[14] Frincke, J. M.; Faulkner, D. J. J. Am. Chem. Soc. 1982, 104, 265.
[15] Parker, K. A.; Cohen, I. D.; Padwa, A.; Dent, W. Tetrahedron Lett. 1984, 25, 4917.
[16] Deblander, J.; Van Aeken, S.; Jacobs, J.; De Kimpe, N. Eur. J. Org. Chem. 2009, 4882.
[17] Claessens, S.; Jacobs, J.; Aeken, S. V.; Tehrani, K. A.; Kimpe, N. D. J. Org. Chem. 2008, 73, 7555.
[18] Pongprom, N.; Bachitsch, H.; Bauchinger, A.; Ettefagh, H.; Haider, T.; Hofer, M.; Knafl, H.; Slanz, R.; Waismeyer, M.; Wieser, F. Monatsh. Chem. 2010, 141, 53.
[19] Huang, H.-M.; Gao, J.-R.; Ye, Q.; Yu, W.-B.; Sheng, W.-J.; Li, Y.-J. RSC Adv. 2014, 4, 15526.
[20] Mckee, T. D.; Suto, R. K. WO 2003073999, 2003.
[21] Gilgen, P.; Jackson, B.; Hansen, H. J.; Heimgartner, H.; Schmid, H. Helv. Chim. Acta 1974, 57, 2634.
[22] Li, Y. J.; Huang, H. M.; Dong, H. Q.; Jia, J. H.; Han, L.; Ye, Q.; Gao, J. R. J. Org. Chem. 2013, 78, 9424.
[23] Lisboa, C. d. S.; Santos, V. G.; Vaz, B. G.; de Lucas, N. C.; Eberlin, M. N.; Garden, S. J. J. Org. Chem. 2011, 76, 5264.
/
| 〈 |
|
〉 |