

Chinese Journal of Organic Chemistry >
Design, Synthesis and Properties of 2/6-Aryl Substituted Azulene Derivatives
Received date: 2018-05-02
Revised date: 2018-06-03
Online published: 2018-06-06
Supported by
Project supported by the National Natural Science Foundation of China (No. 21522209) and the Strategic Priority Research Program (No. XDB12010100).
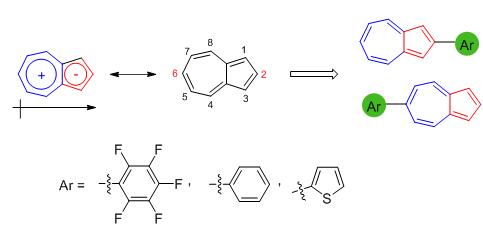
Six 2/6-aryl substituted azulene derivatives 1~6 were designed and synthesised. Compounds 1~3 and 4~6 are 2-and 6-substituted derivatives, respectively, where the arly substituents were pentafluorobenzene, benzene and α-thiophene. The UV-Vis spectra, fluorescence spectra, electrochemical properties and proton-responsive properties of 1~6 were studied. To investigate the molecular sturcture, absorption spectra and energy levels of compounds 1~6, density functional theory (DFT) calculations were carried out. In comparison with the UV-Vis spectra of azulene, the absorption of S0→S2 transition of 1~6 showed red-shift (Δλ=6~68 nm). Owing to the strong electron-donating ability of α-thiophene group, remarkable bathochromic shifts of 3 and 6 (Δλ=68 and 48 nm, respectively) were obseved. The fluorescence spectra revealed that 4 (?F=0.082) has the highest fluorescence quantum yield of 1~6, while 1-H+ (?F=0.359) has the highest fluorescence quantum yield of the protonated compounds 1-H+~6-H+, benefiting from the electron-withdrawing pentafluorophenyl group of 1 and 1-H+. Moreover, the electrochemical analysis and DFT calculations demonstated that the introduction of electron-withdrawing pentafluorophenyl unit in the 2/6-positon of azulene can significantly lower the energy levels of the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO). In comparison with the HOMO/LUMO energy levels of azulene, those of 1 and 4 shift downward with ΔEHOMO/ΔELUMO of -0.23/-0.18 and -0.20/-0.15 eV, respectively. The investigations of physical/chemical properties of 2/6-aryl substituted azulene derivatives will provide valuable insights for developing azulene-based organic functional molecules.
Key words: azulene; aryl substitution; proton response
Gao Honglei , Yang Xiaodi , Xin Hanshen , Gao Tiezhen , Gong Hegui , Gao Xike . Design, Synthesis and Properties of 2/6-Aryl Substituted Azulene Derivatives[J]. Chinese Journal of Organic Chemistry, 2018 , 38(10) : 2680 -2692 . DOI: 10.6023/cjoc201805004
[1] Lemal, D. M.; Goldman, G. D. J. Chem. Educ. 1988, 65, 923.
[2] (a) Michl, J.; Thulstrup, E. W. Tetrahedron 1976, 32, 205.
(b) Dong J.; Zhang H. Chin. Chem. Lett. 2016, 27, 1097.
[3] (a) Rekka, E.; Chrysselis, M.; Siskou, I.; Kourounakis, A. Chem. Pharm. Bull. 2002, 50, 904.
(b) Ishihara, M.; Wakabayashi, H.; Motohashi, N.; Sakagami, H. Anticancer Res. 2011, 31, 515.
[4] Saka, M.; Tsuchikawa, H. JP 04020312, 1992.
[5] Kitamura, Y.; Nakaguchi, T. JP 11125497, 1999.
[6] Yasushige, N.; Shinichi, Y.; Yoshimichi, K.; Seijiro, I.; Satoshi, T. US 20030129516, 2003
[7] (a) Wang, X.; Ng, J. K.-P.; Jia, P.; Lin, T.; Cho, C. M.; Xu, J.; Lu, X.; He, C. Macromolecules 2009, 42, 5534.
(b) Amir, E.; Amir, R. J.; Campos, L. M.; Hawker, C. J. J. Am. Chem. Soc. 2011, 133, 10046.
(c) Koch, M.; Blacque, O.; Venkatesan, K. Org. Lett. 2012, 14, 1580.
(d) Murai, M.; Amir, E.; Amir, R. J.; Hawker, C. J. Chem. Sci. 2012, 3, 2721.
(e) Ghazvini Zadeh, E. H.; Tang, S.; Woodward, A. W.; Liu, T.; Bondar, M. V.; Belfield, K. D. J. Mater. Chem. C 2015, 3, 8495.
(f) Murai, M.; Takami, K.; Takeshima, H.; Takai, K. Org. Lett. 2015, 17, 1798.
[8] Ito, S.; Inabe, H.; Morita, N.; Ohta, K.; Kitamura, T.; Imafuku, K. J. Am. Chem. Soc. 2003, 125, 1669.
[9] (a) Salman, H.; Abraham, Y.; Tal, S.; Meltzman, S.; Kapon, M.; Tessler, N.; Speiser, S.; Eichen, Y. Eur. J. Org. Chem. 2005, 2207.
(b) Zielinski, T.; Kedziorek, M. J.; Jurczak, J. Chem.-Eur. J. 2008, 14, 838.
[10] Cristian, L.; Sasaki, I.; Lacroix, P. G.; Donnadieu, B.; Asselberghs, I.; Clays, K.; Razus, A. C. Chem. Mater. 2004, 16, 3543.
[11] (a) Kurotobi, K.; Kim, K. S.; Noh, S. B.; Kim, D.; Osuka, A. Angew. Chem., Int. Ed. 2006, 45, 3944.
(b) Wang, F. K.; Lin, T. T.; He, C. B.; Chi, H.; Tang, T.; Lai, Y. H. J. Mater. Chem. 2012, 22, 10448.
(c) Ince, M.; Bartelmess, J.; Kiessling, D.; Dirian, K.; Martinez-Diaz, M. V.; Torres, T.; Guldi, D. M. Chem. Sci. 2012, 3, 1472.
[12] (a) Xin, H.; Ge, C.; Gao, H.; Yang, X.; Gao, X. Chem. Sci. 2016, 7, 6701.
(b) Xin, H.; Ge, C.; Jiao, X.; Yang, X.; Rundel, K.; McNeill, C. R.; Gao, X. Angew. Chem., Int. Ed. 2018, 57, 1322.
(c) Xin, H.; Li, J.; Ge, C.; Yang, X.; Xue, T.; Gao, X. Mater. Chem. Front. 2018, 2, 975.
[13] (a) Dutta, S.; Lakshmi, S.; Pati, S. K. Bull. Mater. Sci. 2008, 31, 353.
(b) Dias, J. R. J. Phys. Org. Chem. 2007, 20, 395.
[14] (a) Nozoe, T.; Asao, T.; Oda, M. Bull. Chem. Soc. Jpn. 1974, 47, 681.
(b) Nozoe, T.; Takase, K.; Shimazaki, N., Bull. Chem. Soc. Jpn. 1964, 37, 1644.
[15] (a) Xin, H.; Gao, X. ChemPlusChem 2017, 82, 945.
(b) Koch, M.; Blacque, O.; Venkatesan, K. J. Mater. Chem. C 2013, 1, 7400.
[16] Kim, H.; Schulte, N.; Zhou, G.; Müllen, K.; Laquai, F. Adv. Mater. 2011, 23, 894.
[17] (a) Zhang, J.; Petoud, S. Chem. Eur. J. 2008, 14, 1264.
(b) Nozoe, T.; Takase, K.; Shimazaki, N. Bull. Chem. Soc. Jpn. 1964, 37, 1644.
(c) Nozoe, T.; Seto, S.; Matsumura, S.; Murase, Y. Bull. Chem. Soc. Jpn. 1962, 35, 1179.
(d) McDonald, R. N.; Richmond, J. M.; Curtis, J. R.; Petty, H. E.; Hoskins, T. L. J. Org. Chem. 1976, 41, 1811.
(e)Xin, H.; Ge, C.; Fu, L.; Yang, X.; Gao, X. Chin. J. Org. Chem. 2017, 37, 711(in Chinese). (辛涵申, 葛从武, 傅丽娜, 杨笑迪, 高希珂, 有机化学, 2017, 37, 711.)
[18] Robert, S. H. Liu, J. Chem. Educ. 2002, 79, 183.
[19] (a) Li, L.; Zhang, M. Anal. Chem. 1988, 16, 732(in Chinese). (李隆弟, 张满, 分析化学, 1988, 16, 732.)
(b) Yang, X.; Pan, Z.; Ma, Y. J. Analy. Sci. 2003, 19, 588(in Chinese). (杨洗, 潘祖亭, 马勇, 分析化学, 2003, 19, 588.)
[20] Han, L.; Wu, L.; Tong, Y.; Zu, X.; Jiang, S. Chin. J. Org. Chem. 2017, 37, 2940(in Chinese). (韩亮, 吴靓, 童永正, 祖晓燕, 蒋绍亮, 有机化学, 2017, 37, 2940.)
[21] Lin, D.; Song, S.; Chen, Z.; Guo, P.; Chen, J.; Shi, H.; Mai, Y.; Song, H. Chin. J. Org. Chem. 2018, 38, 103(in Chinese). (林丹燕, 宋森川, 陈智勇, 郭鹏然, 陈江韩, 史华红, 麦裕良, 宋化灿, 有机化学, 2018, 38, 103.)
[22] (a) Shoji, T.; Maruyama, A.; Ito, S.; Okujima, T;; Yasunami, M.; Higashi, J.; Moritae, N. Heterocyles 2014, 89, 2588.
(b) Shoji, T.; Kikuchi, S.; Ito, S.; Morita, N. Heterocyles 2005, 1, 91.
(c) Chen, A.; Chiu, S.; Kuo, Y. Synth. Commun. 2003, 15, 2701.
(d) Kurotobi, K.; Tabata, H.; Miyauchi, M.; Murafuji, T.; Sugihara, Y. Synthesis 2002, 1013.
(e) Jutz, C.; Schweiger, E. Chem. Ber. 1974, 107, 2383.
(f) Shoji, T.; Ito, S.; Toyota, K.; Iwamoto, T.; Yasunami, M.; Morita, N. Eur. J. Org. Chem. 2009, 25, 4307.
/
| 〈 |
|
〉 |