

Chinese Journal of Organic Chemistry >
Synthesis of Chiral α-Amino Acid Derivatives by Asymmetric Addition of α-Imino Ester
Received date: 2018-01-25
Revised date: 2018-04-02
Online published: 2018-06-07
Supported by
Project supported by the National Natural Science Foundation of China (No. 21362025).
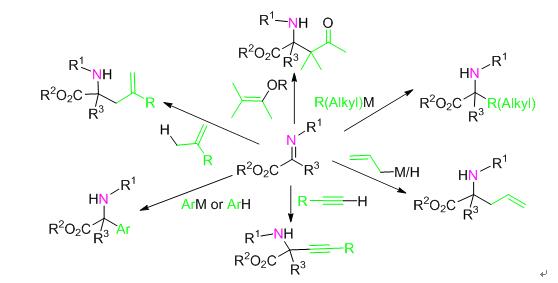
The wide applications of chiral α-amino acid derivatives in the pharmaceutical and fine chemical industry field has greatly arisen the development of its synthetic methods. So far, asymmetric nucleophilic addition reaction of α-imino ester has been proven to be one of the most effective methods to synthesize chiral α-amino acid derivatives and has been focused by chemists in the field of asymmetric catalysis. The development of such method on the view of reaction types and different kinds of nucleophiles is described. Specifically, allylation reaction, arylation reactions, Mannich reactions, alkenylation reactions, alkynylation reactions and alkylation reactions are introduced, together with the associated reaction mechanisms and recent developments. Additionally, a prospect on this research field is given.
Bi Jili , Ma Ransong , Yang Jinhui . Synthesis of Chiral α-Amino Acid Derivatives by Asymmetric Addition of α-Imino Ester[J]. Chinese Journal of Organic Chemistry, 2018 , 38(10) : 2553 -2570 . DOI: 10.6023/cjoc201801035
[1] (a) Salih, N.; Adams, H.; Jackson, R. J. Org. Chem. 2016, 81, 8386.
(b) Chen, Y.-Y.; Chang, L.-T.; Chen, H.-W.; Yang, C.-Y.; Hsin, L.-W. ACS. Comb. Sci. 2017, 19, 131.
(c) Vanda, D.; Jorda, R.; Lemrová, B.; Volná, T.; Krytsof, V.; McMaster, C.; Soural, M. ACS Comb. Sci. 2015, 17, 426.
(d) Jarvo, E. R.; Miller, S. J. Tetrahedron 2002, 58, 2481.
(e) Kazmaier, U. Angew. Chem., Int. Ed. 2005, 44, 3509.
(f) Prabhu, G.; Narendra, N.; Pandurangaa, V.; Sureshbabu,V. V.; RSC Adv. 2015, 5, 48331.
[2] Wei, Q.-L.; Zhang, F.; Zhao, X.-F.; Wang, C.; Xiao, J.-L.; Tang.W.-J. Org. Biomol. Chem. 2017, 15, 5468.
[3] (a) Tararov, V. I.; Borner, A. Synlett 2005, 203.
(b) Tararov, V. I.; Kadyrov, R.; Riermeier, T. H.; Borner, A. Chem. Commun. 2000, 1867.
(c) Kadyrov, R.; Riermeier, T. H.; Dingerdissen, U.; Tararov, V.; Borner, A. J. Org. Chem. 2003, 68, 4067.
[4] (a) Avenoza, A.; Busto, J. H.; Peregrina, J. M.; Pérez-Fernández, M. Tetrahedron 2005, 61, 4165.
(b) Avenoza, A.; Busto, J. H.; Canal, N.; Peregrina, J. M.; Pérez-Fernández, M. Org. Lett. 2005, 7, 3597.
(c) Akiyama, T.; Daidouji, K.; Fuchibe, K. Org. Lett. 2003, 5, 3691.
(d) Yao, S.; Saaby, S.; G.Hazell, R.; Johannsen, K. A. Chem.-Eur. J. 2000, 6, 2435.
[5] (a) Chen, D.; Xu, M.-H. Chin. J. Org. Chem. 2017, 37, 1589(in Chinese). (陈雕, 徐明华, 有机化学, 2017, 37, 1589.)
(b) Bian, Q.-H.; Zhong, J.-C.; Hou, S.-C.; Wang, M. Chin. J. Org. Chem. 2010, 30, 1261(in Chinese). (边庆花, 钟江春, 侯士聪, 王敏, 有机化学, 2010, 30, 1261.)
(c) Cheng, Y.-H.; Zou, X.-M.; Wu, C.; Yang, H.-Z. Chin. J. Org. Chem. 2001, 30, 1(in Chinese). (程永浩, 邹小毛, 吴超, 杨华铮, 有机化学, 2001, 30, 1.)
(d) Li, Z.-J.; Wan, G.-H.; Wei, P.; Shi, Y.-H.; Ou-yang, P.-K. Chin. J. Org. Chem. 2005, 25, 881(in Chinese). (李振江, 万红贵, 韦萍, 石玉瑚, 欧阳平凯, 有机化学, 2005, 25, 881.)
(e) Lin, J.; Fan, H.-D.; Yan, S.-J. Chin. J. Org. Chem. 2007, 27, 925(in Chinese). (林军, 樊会丹, 严胜骄, 有机化学, 2007, 27, 925.)
(f) Lin, J. Chin. J. Org. Chem. 2009, 24, 33(in Chinese). (刘静, 有机化学, 2009, 24, 33.)
[6] Eftekhari-Sis, B.; Zirak. M. Chem. Rev. 2017, 117, 8326.
[7] Liu, M.; Shen, A.; Sun, X.-W.; Deng, F.; Xu, M.-H.; Lin, G.-Q. Chem. Commun. 2010, 46, 8460.
[8] Ferraris, D.; Young, B.; Cox, C.; Dudding, T.; Drury, W. J.; Ryzhkov, L.; Taggi, A. E.; Lectka, T. J. Am. Chem. Soc. 2002, 124, 67.
[9] Hamada, T.; Manabe, K.; Kobayashi, S. Angew. Chem., Int. Ed. 2003, 42, 3927.
[10] Ogawa, C.; Sugiura, M.; Kobayashi, S. Angew. Chem., Int. Ed. 2004, 43, 6491.
[11] Fang, X.-M.; Johannsen, M.; Yao, S.; Gathergood, N.; Hazell, R. G.; Jørgensen, K. A. J. Org. Chem. 1999, 64, 4844.
[12] Colombo, F.; Annunziata, R.; Benaglia, M. Tetrahedron. Lett. 2007, 48, 2687.
[13] Fujita, M.; Nagano, T.; Schneider, U.; Hamada, T.; Ogawa, C.; Kobayashi, S. J. Am. Chem. Soc. 2008, 130, 2914.
[14] Beenen, M. A.; Weix, D. J.; Ellman, J. A. J. Am. Chem. Soc. 2006, 128, 6304.
[15] Dai, H.-X.; Lu, X.-Y. Org. Lett. 2007, 9, 3077.
[16] Ji, D.-M.; Xu, M.-H. Chem. Commun. 2010, 46, 1550.
[17] Chen, J.-Y.; Lu, X.-X.; Lou, W.-Y.; Ye, Y.; Jiang, H.-F.; Zeng, W. J. Org. Chem. 2012, 77, 8541.
[18] Li, Y.; Yu, Y.-N.; Xu, M.-H. ACS Catal. 2016, 6, 661.
[19] Takechi, R.; Nishimura, T. Org. Biomol. Chem. 2015, 13, 4918.
[20] Zhou, B.; Li, K.-Z.; Jiang, C.-H.; Lu, Y.-X.; Hayashi, T. Adv. Synth. Catal. 2017, 359, 1.
[21] Johannsen, M. Chem. Commun. 1999, 2233.
[22] Saaby, S.; Fang, X.-M.; Gathergood, N.; Johannsen, K. A. Angew. Chem., Int. Ed. 2000, 39, 4114.
[23] Churches, Q. I.; White, J. M.; Hutton, C. A. Org. Lett. 2011, 13, 2900.
[24] Li, Y.; Xu, M.-H. Org. Lett. 2012, 14, 2062.
[25] Sugiyama, S.; Imai, S.; Ishii, K. Tetrahedron:Asymmetry 2013, 24, 1069.
[26] Hagiwara, E.; Fujii, A.; Sodeoka, M. J. Am. Chem. Soc. 1998, 120, 2474.
[27] Ferraris, D.; Young, B.; Cox, C.; Dudding, T.; Drury Ⅲ, W. J.; Ryzhkov, L.; Taggi, A. E.; Lectka, T. J. Am. Chem. Soc. 2002, 124, 67.
[28] Ferraris, D.; Young, B.; Dudding, T.; Lectka, T. J. Am. Chem. Soc. 1998, 120, 4548.
[29] Kobayashi, S.; Matsubara, R.; Nakamura, Y.; Kitagawa, H.; Sugiura, M. J. Am. Chem. Soc. 2003, 125, 2507.
[30] Hamada, T.; Manabe, K.; Kobayashi, S. J. Am. Chem. Soc. 2004, 126, 7768.
[31] (a) Xie, L.; Ma, H.-L.; Li, J.-Q.; Yu, Y.; Qin, Z.-H.; Fu, B. Org. Chem. Front. 2017, 4, 1858.
(b) Yu, J.-S.; Zhou, J. Org. Chem. Front. 2016, 3, 298.
[32] Zhang, H.; Syed, S.; Barbas Ⅲ, C. F. Org. Lett. 2010, 12, 708.
[33] Perera, S.; Sinha, D.; Rana, N. K.; Trieu-Do, V.; Zhao, C.-G. J. Org. Chem. 2013, 78, 10947.
[34] Valero, G.; León, C. M.; Moyano, A. Asymmetric Catal. 2015, 2, 7.
[35] Liu, X.-D.; Deng, L.-J.; Jiang, X.-X.; Yan, W.-J.; Liu, C.-L.; Wang, R. Org. Lett. 2010, 12, 876.
[36] Wu, L.; Li, G.-X.; He, M.-G.; Wang, Y.-W.; Zhao, G.; Tang, Z. Can. J. Chem. 2016, 94, 769.
[37] Tao, Z.-L.; Adele, A.; Wu, X.; Gong, L.-Z. Chin. J. Chem. 2014, 32, 969.
[38] Veverková, E.; Liptáková, L.; Veverka, M.; Sebesta, R. Tetrahedron:Asymmetry 2013, 24, 548.
[39] Hernández-Toribio, J.; Arrayás, R. G.; Carretero, J. C. Chem.-Eur. J. 2010, 16, 1153.
[40] (a) Veverková, E.; Strasserová, J.; Sebesta, R.; Toma, S. Tetrahedron:Asymmetry 2010, 21, 58.
(b) Lu, N.; Fang, Y.-H.; Gao, Y.; Wei, Z.-L.; Cao, J.-G.; Liang, D.-P.; Lin, Y.-J.; Duan, H.-F. J. Org. Chem. 2018, 83, 1486.
(c) Wang, Y.-H.; Liu, Y.-L.; Cao, Z.-Y.; Zhou, J. Asian J. Org. Chem. 2014, 3, 429.
[41] Matsubara, R.; Nakamura, Y.; Kobayashi, S. Angew. Chem., Int. Ed. 2004, 43, 1679.
[42] Juhl, K.; Gathergood, N.; Jørgensen, K. A. Angew. Chem., Int. Ed. 2001, 40, 2995.
[43] Bernardi, L.; Gothelf, A. S.; Hazell, R. G.; Jørgensen, K. A. J. Org. Chem. 2003, 68, 2583.
[44] Yang, C.-F.; Shen, C.; Wang, J.-Y.; Tian, S.-K. Org. Lett. 2012, 14, 3092.
[45] Marigo, M.; Kjærsgaard, A.; Juhl, K.; Gathergood, N.; Jørgensen, K. A. Chem.-Eur. J. 2003, 9, 2359.
[46] Foltz, C.; Stecker, B.; Marconi, G.; Bellemin-Laponnaz, S.; Wadepohla, H.; Gade, L. H. Chem. Commun. 2005, 5115.
[47] Kjærsgaard, A.; Jørgensen, K. A. Org. Biomol. Chem. 2005, 3, 804.
[48] Trost, B. M.; Terrell, L. R. J. Am. Chem. Soc. 2003, 125, 338.
[49] Ooi, T.; Kameda, M.; Fujii, J.-I.; Maruoka, K. Org. Lett. 2004, 6, 2397.
[50] Córdova, A.; Notz, W.; Zhong, G.-F.; Betancort, J. M.; Barbas Ⅲ, C. F. J. Am. Chem. Soc. 2002, 124, 1842.
[51] Wasa, M.; Liu, R. Y.; Roche, S. P.; Jacobsen, E. N. J. Am. Chem. Soc. 2014, 136, 12872.
[52] Yu, J.-S.; Zhou, J. Org. Chem. Front. 2016, 3, 298.
[53] Lou, S.; Schaus, S. E. J. Am. Chem. Soc. 2008, 130, 6922.
[54] Drury Ⅲ, W. J.; Ferraris, D.; Cox, C.; Young, B.; Lectka, T. J. Am. Chem. Soc. 1988, 120, 11006.
[55] Yao, S.; Fang, X.-M.; Jørgensen, K. A. Chem. Commun. 1998, 2547.
[56] Caplan, N. A.; Hancock, F. E.; Bulman Page, P. C.; Hutchings, G. J. Angew. Chem., Int. Ed. 2004, 43, 1685.
[57] Liu, R.-R.; Wang, D.-J.; Wu, L.; Xiang, B.; Zhang, G.-Q.; Gao, J.-R.; Jia, Y.-X. ACS Catal. 2015, 5, 6524.
[58] Shao, Z.-H.; Wang, J.; Ding, K.; Chan, A. S. C. Adv. Synth. Catal. 2007, 349, 2375.
[59] Peng, F.-Z.; Shao, Z.-H.; Chan, A. S. C. Tetrahedron:Asymmetry 2010, 21, 465.
[60] Rueping, M.; Antonchick, A. P.; Brinkmann, C. Angew. Chem., Int. Ed. 2007, 46, 6903.
[61] Huang, G.-C.; Yang, J.; Zhang, X.-G. Chem. Commun. 2011, 47, 5587.
[62] Zhang, F.-G.; Ma, H.; Zheng, Y.; Ma, J.-A. Tetrahedron 2012, 68, 7663.
[63] Morisaki, K.; Sawa, M.; Nomaguchi, J.-Y.; Morimoto, H.; Takeuchi, Y.; Mashima, K.; Ohshima, T. Chem.-Eur. J. 2013, 19, 8417.
[64] Chiev, K.-P.; Roland, S.; Mangeney, P. Tetrahedron:Asymmetry 2002, 13, 2205.
[65] Hatano, M.; Yamashita, K.; Mizuno, M.; Ito, O.; Ishihara, K. Angew. Chem., Int. Ed. 2015, 127, 2745.
/
| 〈 |
|
〉 |