

Chinese Journal of Organic Chemistry >
Asymmetric Synthesis of the Tetracyclic Skeleton of Natural Product Arborisidine
Received date: 2018-05-10
Revised date: 2018-06-04
Online published: 2018-06-15
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21572140, 21732005).
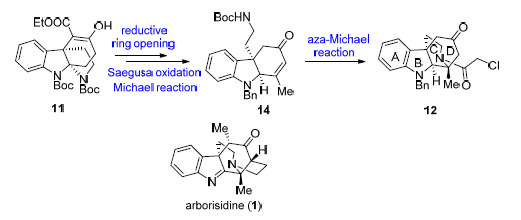
Asymmetric synthesis of the tetracyclic skeleton of arborisidine is reported. Starting from the enantiomerically pure compound 10,9-di-tert-butyl5-ethyl(4S,8R)-6-hydroxy-7,8-dihydro-9H-8a,4b-(epiminoethano)carbazole-5,9,10-tricar-boxylate (11) which was reported earlier by our group, a Krapcho decarboxylation reaction was used to afford the ketone, and then a reductive ring-opening method was applied to open the pyrrolidine ring of the substrate. The C(15)-C(16) double bond and the methyl group at C(16) of A/B/D tricyclic skeleton were introduced via Saegusa oxidation and Michael reaction, respectively. Finally, an intramolecular aza-Michael addition reaction was used as a key reaction to construct the C-ring and C(16) quaternary center, which led to the efficiently asymmetric synthesis of A/B/C/D tetracyclic skeleton of arborisidine.
Chen Zhitao , Xiao Tao , Song Hao , Qin Yong . Asymmetric Synthesis of the Tetracyclic Skeleton of Natural Product Arborisidine[J]. Chinese Journal of Organic Chemistry, 2018 , 38(9) : 2427 -2434 . DOI: 10.6023/cjoc201805025
[1] Wong, S.-P.; Chong, K.-W.; Lim, K.-H.; Lim, S.-H.; Low, Y.-Y.; Kam, T.-S. Org. Lett. 2016, 18, 1618.
[2] Wong, S.-P.; Gan, C.-Y.; Lim, K.-H.; Ting, K.-N.; Low, Y.-Y.; Kam, T.-S. Org. Lett. 2015, 17, 3628.
[3] Subramaniam, G.; Hiraku, O.; Hayashi, M.; Koyano, T.; Komiyama, K.; Kam, T.-S. J. Nat. Prod. 2007, 70, 1783.
[4] Schnoes, H. K.; Biemann, K.; Mokry, J.; Kompis, L.; Chatterjee, A.; Ganguli, G. J. Org. Chem. 1966, 31, 1641.
[5] Li, L.; Yang, T.; Liu, Y.; Liu, J.; Li, M.; Wang, Y.; Yang, S.; Zou, Q.; Li, G. Org. Lett. 2012, 14, 3450.
[6] Gan, P.; Pitzen, J.; Qu, P.; Snyder, S. A. J. Am. Chem. Soc. 2018, 140, 919.
[7] (a) Zu, L.; Boal, B. W.; Garg, N. K. J. Am. Chem. Soc. 2011, 133, 8877.
(b) Ren, W.; Wang, Q.; Zhu, J. Angew. Chem., Int. Ed. 2014, 53, 1818.
(c) Teng, M.; Zi, W.; Ma, D. Angew. Chem., Int. Ed. 2014, 53, 1814.
[8] (a) Moreno, J.; Picazo, E.; Morrill, L. A.; Smith, J. M.; Garg, N. K. J. Am. Chem. Soc. 2016, 138, 1162.
(b) Jiang, S.-Z.; Zeng, X.-Y.; Liang, X.; Lei, T.; Wei, K.; Yang, Y.-R. Angew. Chem., Int. Ed. 2016, 55, 4044.
(c) Wang, T.; Duan, X.; Zhao, H.; Zhai, S.; Tao, C.; Wang, H.; Li, Y.; Cheng, B.; Zhai, H. Org. Lett. 2017, 19, 1650.
[9] Moreno, J.; Picazo, E.; Morrill, L. A.; Smith, J. M.; Garg, N. K. J. Am. Chem. Soc. 2016, 138, 1162.
[10] Ren, W.; Wang, Q.; Zhu, J. Angew. Chem., Int. Ed. 2016, 55, 3500.
[11] (a) Xie, X.; Wei, B.; Li, G.; Zu, L. Org. Lett. 2017, 19, 5430.
(b) Li, G.; Xie, X.; Zu, L. Angew. Chem., Int. Ed. 2016, 55, 10483.
[12] Nishiyama, D.; Ohara, A.; Chiba, H.; Kumagai, H.; Oishi, S.; Fujii, N.; Ohno, H. Org. Lett. 2016, 18, 1670.
[13] (a) Eckermann, R.; Breunig, M.; Gaich, T. Chem. Commun. 2016, 52, 11363.
(b) Eckermann, R.; Breunig, M.; Gaich, T. Chem.-Eur. J. 2017, 23, 3938.
[14] Smith, M. W.; Zhou, Z.; Gao, A. X.; Shimbayashi, T.; Snyder, S. A. Org. Lett. 2017, 19, 1004.
[15] (a) Xiao, T.; Chen, Z.-T.; Deng, L.-F.; Zhang, D.; Liu, X.-Y.; Song, H.; Qin, Y. Chem. Commun. 2017, 53, 12665.
(b) Chen, Z.-T.; Xiao, T.; Tang, P.; Zhang, D.; Qin, Y. Tetrahedron 2018, 74, 1129.
[16] Zhang, D.; Song, H.; Qin, Y. Acc. Chem. Res. 2011, 44, 447
[17] Shen, L.; Zhang, M.; Wu, Y.; Qin, Y. Angew. Chem., Int. Ed. 2008, 47, 3618.
[18] Zhang, M.; Huang, X.; Shen, L.; Qin, Y. J. Am. Chem. Soc. 2009, 131, 6013.
[19] (a) Eckermann, R.; Gaich, T. Synthesis 2013, 45, 2813.
(b) Smith, J. M.; Moreno, J.; Boal, B. W.; Garg, N. K. Angew. Chem., Int. Ed. 2015, 54, 400.
(c) Adams, G, L; Smith, A. B. The Alkaloids. Chemistry and Biology, Elsevier, New York, 2016, 76, 171.
/
| 〈 |
|
〉 |