

Chinese Journal of Organic Chemistry >
Synthesis and Bioactivities of Novel 1,2,4-Triazine Scheleton Phenanthroline Derivatives and the Fluorescent Recognition on DNA Using Three Novel Co (III) Complexes
Received date: 2018-03-28
Revised date: 2018-06-07
Online published: 2018-06-29
Supported by
Project supported by the Science and Technology Research Program of Liaoning Provincial Department of Education (No. 2009A426).
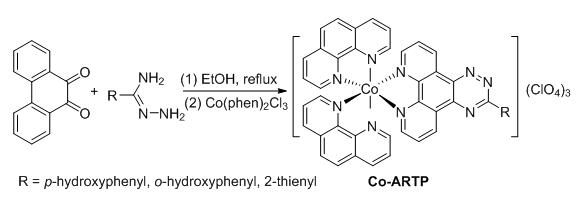
Cdc25B has become the important target for curing cancer owing to its over expression in kinds of cancers. Compounds containing phenanthroline moiety have become research objectives on the DNA fluorescence probes or the new curing agents for their excellent fluorescence property and bioactivities. Twelve novel 1,2,4-triazine scheleton phenanthroling derivatives ARTP1~ARTP12 were first designed and synthesized, the structures of ARTP1~ARTP12 were characterized successfully by means of IR and NMR. The inhibitory activities of ARTP1~ARTP12 against Cdc25B were evaluated. The results show that nine target molecules exhibit excellent inhibitories, four molecules behave better activities than the contrast reference Na3VO4 indicating that they may be used as Cdc25B inhibitors. Meanwhile, three novel complexes Co-ARTP-5, Co-ARTP-6 and Co-ARTP-10 were first afforded by the reaction of the excellent inhibitory active compounds ARTP5, ARTP6 and ARTP10 with Co3+ respectively. The structures of the three complexes were confirmed through IR, UV-Vis, 1H NMR and fluorescence spectra. The interaction modes between the complexes and CT-DNA were explored. As a result, the excitation peaks of the complexes show a red shift and the complexes interact with CT-DNA through the insert mode. The binding constants Kb are (2.12±0.20)×105, (3.29±0.20)×105and (1.50±0.20)×105 L·mol-1, respectively, and it occurs strong fluorescene quenching. The complexes are expected to be the DNA fluorescence probes.
Key words: 1,10-phenanthroline; 1,2,4-triazine; Cdc25B; Co(III) complex; fluorescent
Zhang Chenglu , Li Yizheng , Li Jingyi , Li Yilin , Gong Rongqing , Wang Huayu . Synthesis and Bioactivities of Novel 1,2,4-Triazine Scheleton Phenanthroline Derivatives and the Fluorescent Recognition on DNA Using Three Novel Co (III) Complexes[J]. Chinese Journal of Organic Chemistry, 2018 , 38(10) : 2720 -2730 . DOI: 10.6023/cjoc201803048
[1] Mak, L. H.; Knott, J.; Scott, K. A.; Scott, C.; Whyte, G. F.; Ye, Y.; Mann, D. J.; Ces, O.; Stivers, J.; Woscholski, R. Bioorg. Med. Chem. 2012, 20, 4371.
[2] He, X. P.; Deng, Q.; Gao, L. X.; Li, C.; Zhang, W.; Zhou, Y. B.; Tang, Y.; Shi, X. X.; Xie J.; Li J.; Chen, G. R.; Chen, K. X. Bioorg. Med. Chem. 2011, 19, 3892.
[3] Sarkis, M.; Tran, D. N.; Kolb, S.; Miteva, M. A.; Villoutreix, B. O.; Garbay, C.; Braud, E. Bioorg. Med. Chem. Lett. 2012, 22, 7345.
[4] Bäurle, S.; Blume, T.; Günther, J.; Henschel, D.; Hillig, R. C.; Husemann, M.; Mengel, A.; Parchmann, C.; Schmid, E.; Skuballa, W. Bioorg. Med. Chem. Lett. 2004, 14, 1673.
[5] Barton, J. K.; Danishefsky, A.; Goldberg, J. J. Am. Chem. Soc. 1984, 106, 2172.
[6] Demeunynck, M.; Bailly, C.; Wilson, W. D. DNA and RNA Binders, From Small Molecules to Drugs, Vol. 1, Wiley-VCH, Weinheim, 2003.
[7] Masood, M. A.; Hodgson, D. J. Inorg. Chem. 1993, 32, 4839.
[8] Kalyanasundaram, K.; Gratzel, M. Coord. Chem. Rev. 1998, 77, 347.
[9] Barton, J. K.; Danishefsky, A. T.; Goldberg, J. M. J. Am. Chem. Soc. 1984, 106, 2172.
[10] Juris, A.; Balzani, V.; Barigelletti, F.; Campagna, S.; Belser, P.; Von Zelewsky, A. Coord. Chem. Rev. 1988, 84, 85.
[11] Balzani, V.; Juris, A.; Venturi, M.; Campagna, S.; Serroni, S. Chem. Rev. 1996, 96, 759.
[12] Cola, L. D.; Belser, P. Coord. Chem. Rev. 1998, 177, 301.
[13] Kaes, C.; Katz, A.; Hosseini, M. W. Chem. Rev. 2000, 100, 3553.
[14] Chao, H.; Ji, L. N. Bioinorg. Chem. Appl. 2005, 3, 15.
[15] Vos, J. G.; Kelly, J. M. J. Chem. Soc., Dalton Trans. 2006, 41, 4869.
[16] Tan, L. F.; Liu, J. H.; Wang, F.; Zhang, S. Chem. Biodiversity 2007, 4, 2863.
[17] Liu, Y. J.; Wang, N.; Mei, W. J.; Chen, F.; He, L. X.; Jian, L. Q.; Wang, R. J. Transition. Met. Chem. 2007, 32, 332.
[18] Xu, H.; Deng, H.; Zhang, Q. L.; Huang, Y.; Liu, J. Z.; Li, L. L. Inorg. Chem. Commun. 2003, 6, 766.
[19] Tan, L. F.; Chao, H.; Liu, Y. J.; Li, H.; Sun, B.; Ji, L. N. Inorg. Chim. Acta 2005, 358, 2191.
[20] Cédric, M. R.; Eddy, D.; Francis, S. Chem. Commun. 2005, 345.
[21] Mahajan, S. S.; Scian, M.; Sripathy, S.; Posakony, J.; Lao, U.; Loe, T. K.; Leko, V.; Thalhofer, A.; Schuler, A. D.; Bedalov, A.; Simon, J. A. J. Med. Chem. 2014, 57, 3283.
[22] Wang, Z. N.; Xue, S. J. Chin. J. Org. Chem. 2002, 22, 174(in Chinese). (王仲南, 薛思佳, 有机化学, 2002, 22, 174.)
[23] Paul, K.; Sharma, A.; Luxami, V. Bioorg. Med. Chem. Lett. 2014, 24, 624.
[24] Khoshneviszadeh, M.; Ghahremani, M. H.; Foroumadi, A.; Miri, R.; Firuzi, O.; Madadkar-Sobhani, A.; Edraki, N.; Parsa, M.; Shafiee, A. Bioorg. Med. Chem. 2013, 21, 6708.
[25] El-Sayed Ali, T. S. Eur. J. Med. Chem. 2009, 44, 4385.
[26] Stefek, M.; Soltesova Prnova, M.; Majekova, M.; Rechlin, C.; Heine, A.; Klebe, G. J. Med. Chem. 2015, 58, 2649.
[27] Luo, R.; Liu, L.; Si, Y.; Fang, J. J.; Xu, H. R.; Zhao, L. L.; An, D. M.; Mu, J. Mod. Prev. Med. 2011, 38, 4550(in Chinese). (罗榕, 刘凌, 司洋, 方嘉佳, 徐鸿儒, 赵丽莉, 安冬梅, 慕洁, 现代预防医学, 2011, 38, 4550.)
[28] Guo, X. X.; Du, X. X.; Xue, S. S. Chin. J. Pharmacovigilance 2011, 8, 212(in Chinese). (郭晓昕, 杜晓曦, 薛松林, 中国药物警戒, 2011, 8, 212.)
[29] Li, J.; Li, X. M.; Wang, H. Y. Int. J. Intern. Med. 1999, 26, 107(in Chinese). (李健, 李晓玫, 王海燕, 国外医学内科学分册, 1999, 26, 107.)
[30] Cui, X. Y.; Cao, M. F.; Sun, H.; Yu, B. L. Med. Lab. Sci. Clin. 2008, 16, 71(in Chinese). (崔秀玉, 曹美芳, 孙华, 于保玲, 医学检验与临床, 2008, 16, 71.)
[31] Sangshetti, J. N.; Shinde, D. B. Bioorg. Med. Chem. Lett. 2010, 20, 742.
[32] Misra, U.; Hitkari, A.; Saxena, A. K.; Gurtu, S.; Shanker, K. Eur. J. Med. Chem. 1996, 31, 629.
[33] Lindsley, C. W.; Wisnoski, D. D.; Wang, Y.; Leister, W. H.; Zhao, Z. J. Tetrahedron Lett. 2003, 44, 4495.
[34] Thirumurugan, P.; Perumal, P. T. Dyes Pigm. 2011, 88, 403.
[35] Ghazvini Zadeh, E. H.; El-Gendy, B. E. M.; Popa, A. G.; Katritzky, A. R. Med. Chem. Commun. 2012, 3, 52.
[36] Chai, J. H.; Wang, Y.; Xu, D. Q.; Wang, X.; Zhu, C. A.; Guo, Y.; Zhang, C. L. Chem. J. Chin. Univ. 2014, 35, 750(in Chinese). (柴金华, 王越, 徐德青, 王雪, 朱长安, 国阳, 张成路, 高等学校化学学报, 2014, 35, 750.)
[37] Zhang, C. L.; Wang, X.; Guo, Y.; Wu, Y. F.; Gao, L. N.; Sun, L. J. Chin. J. Org. Chem. 2014, 34, 2331(in Chinese). (张成路, 王雪, 国阳, 吴一非, 高丽娜, 孙立杰, 有机化学, 2014, 34, 2331.)
[38] Zhu, C. A.; Wu, F. Y.; Wang, X.; Gao, L. N.; Weng, Q. F.; Shi, L.; Zhang, C. L. Chin. J. Appl. Chem. 2014, 31, 455(in Chinese). (朱长安, 武飞宇, 王雪, 高丽娜, 翁前锋, 石丽, 张成路, 应用化学, 2014, 31, 455.)
[39] Zhang, C. L.; Guo, Y.; Chen, Y.; Sun, L. J.; Cheng, A. Q.; Zhao, N.; Zhao, B. C.; Tang, J.; Xi, H. Chin. J. Org. Chem. 2015, 35, 1665(in Chinese). (张成路, 国阳, 陈莹, 孙立杰, 程安琪, 赵娜, 赵宝成, 唐杰, 袭焕, 有机化学, 2015, 35, 1665.)
[40] Shui, Y. H. J. Chengdu Text. Coll. 2000, 17, 61(in Chinese). (税永红, 成都纺织高等专科学校学报, 2000, 17, 61.)
[41] Wang, G. Z.; Wang, Q. X. Stud. Trace Elem. Health 2004, 21, 54(in Chinese). (王根志, 王秋霞, 微量元素与健康研究, 2004, 21, 54.)
[42] Park, H.; Bahn, Y. H.; Jung, S. K.; Jeong, D. G.; Lee, S. H.; Seo, I.; Yoon, T. S.; Kim, S. J.; Ryu, S. E. J. Med. Chem. 2008, 51, 5533.
[43] Vlcek, A. A. Inorg. Chem. 1967, 6, 1425.
[44] Heinrich W, Milena T. Collect. Czech. Chem. Commun. 2003, 68, 965.
[45] Akritopoulou-Zanze, I.; Wang, Y.; Zhao, H.; Djuric, S. W. Tetrahedron Lett. 2009, 50, 5773.
[46] Zou, X. H.; Ye, B. H.; Li, H.; Liu, J. G.; Xiong, Y.; Ji, L. N. J. Chem. Soc., Dalton Trans. 1999, 9, 1423.
[47] Pabst, G. R.; Pfüller, O. C.; Sauer, J. Tetrahedron Lett. 1998, 39, 8825.
[48] Chao, H.; Qiu, Z. R.; Cai, L. R.; Zhang, H.; Li, X. Y.; Wong, K. S.; Ji, L. N. Inorg. Chem. 2003, 42, 8823.
/
| 〈 |
|
〉 |