

Chinese Journal of Organic Chemistry >
CBr4-Promoted Efficient Synthesis of 2-(1H-Benzo[d] imidazol-2-yl)-3-arylacrylonitriles
Received date: 2018-04-18
Revised date: 2018-06-13
Online published: 2018-06-29
Supported by
Project supported by the National Natural Science Foundation of China (No. 51403073), the Department of Education of Jiangsu Province (No. 16KJB150006) and the Jiangsu Key Laboratory for Chemistry of Low-Dimensional Materials (No. JSKC15145).
2-(1H-Benzo[d] imidazol-2-yl)-3-arylacrylonitrile derivatives not only exhibit a variety of important biological activities, but also are important intermediates in organic synthesis. The CBr4-promoted reaction of aromatic aldehydes with 2-(1H-benzo[d] imidazol-2-yl) acetonitrile to obtain 2-(1H-benzo[d] imidazol-2-yl)-3-arylacrylonitriles was developed. Structurally diverse 2-(1H-benzo[d] imidazol-2-yl)-3-arylacrylonitriles were obtained in moderate to good yields (74%~96%) under mild conditions. This method has the advantages of operational simplicity and wide substrate scope.
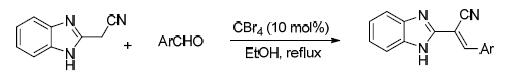
Key words: benzimidazol; carbon tetrabromide; acrylonitrile; Knoevenagel condensation
Wang Xiang , Chen Ping , Zhi Sanjun , Hu Huayou , Kan Yuhe . CBr4-Promoted Efficient Synthesis of 2-(1H-Benzo[d] imidazol-2-yl)-3-arylacrylonitriles[J]. Chinese Journal of Organic Chemistry, 2018 , 38(11) : 3123 -3126 . DOI: 10.6023/cjoc201804037
[1] (a) Yu, N.; Aramini, J. M.; Germann, M. W.; Huang, Z. Tetrahedron Lett. 2000, 41, 6993.
(b) Hou, H.; Li, Z.; Ying, A.; Xu, S. Chin. J. Org. Chem. 2014, 34, 1277(in Chinese). (侯海亮, 李志峰, 应安国, 许松林, 有机化学, 2014, 34, 1277.)
[2] Tietze, L. F.; Rackelmann, N. Pure Appl. Chem. 2004, 76, 1967.
[3] (a) Kozaki, M.; Isoyama, A.; Kogen, A. A.; Okada, K. Org. Lett. 2005, 7, 115.
(b) Krebs, F. C.; Spanggaard, H. J. Org. Chem. 2002, 67, 7185.
[4] Li, Q.; Xiang, S.; Mao, S.; Ren, Y. Chin. J. Org. Chem. 2017, 37, 608(in Chinese). (李强根, 向仕凯, 毛双, 任译, 有机化学, 2017, 37, 608.)
[5] Chen, Z.; Liu, X.-K.; Zheng, C.-J.; Ye, J.; Liu, C.-L.; Li, F.; Ou, X.-M.; Lee, C.-S.; Zhang, X.-H. Chem. Mater. 2015, 27, 5206.
[6] Oksana, T.; Nicolas, M.; Chiara, B. J. Mater. Chem. C 2016, 4, 5940.
[7] Tmur, G.; Andre, F.; Teulon J.-M.; Daniel, P.; Michele, C.; Alix, C. J. Med. Chem. 1992, 35, 4455.
[8] Hasegawa, M.; Nishigaki, N.; Washio, Y.; Kano, K.; Harris, P. A.; Sato, H.; Mori, I.; West, R. I.; Shibahara, M.; Toyoda, H.; Wang, L.; Nolte, R. T.; Veal, J. M.; Cheung, M. J. Med. Chem. 2007, 50, 4453.
[9] Refaat, H. M. Eur. J. Med. Chem. 2010, 45, 2949.
[10] Hranjec, M.; Pavlovic, G.; Marjanovic, M.; Kralj, M.; Karminski-Zamola, G. Eur. J. Med. Chem. 2010, 45, 2405.
[11] Mugnaini, C.; Rajamaki, S.; Tintori, C.; Corelli, F.; Massa, S.; Witvrouw, M.; Debyser, Z.; Veljkovic, V.; Botta, M. Bioorg. Med. Chem. Lett. 2007, 17, 5370.
[12] Hsu, W.-S.; Tsai, M.-H.; Barve, I. J.; Yellol, G. S.; Sun, C.-M. ACS Comb. Sci. 2017, 19, 492.
[13] Panda, K.; Suresh, J. R.; Ila, H.; Junjappa, H. J. Org. Chem. 2003, 68, 3498.
[14] Narhe, B. D.; Tsai, M.-H.; Sun, C.-M. ACS Comb. Sci. 2014, 16, 421.
[15] (a) Gao, X.; Gao, C.; Gao, R. Asian J. Chem. 2015, 27, 2145.
(b) Srinivas, K.; Katari, N. K.; Karuna, P. S.; Surendra Babu, M. S. Curr. Organocatal. 2015, 2, 44.
(c) Hranjec, M.; Karminski-Zamola, G. Molecules 2007, 12, 1817.
[16] Dubey, P. K.; Reddy, P. V. V. P. Indian J. Heterocycl. Chem. 2007, 16, 395.
[17] Kazi, I.; Guha, S.; Sekar, G. Org. Lett. 2017, 19, 1244.
[18] Kishore Babu, P. N.; Rama Devi, B.; Dubey, P. K. Chem. Sin. 2013, 4, 107.
[19] Saczewski, F.; Reszka, P; Gdaniec, M; Gruenert, R; Bednarski, P. J. J. Med. Chem. 2004, 47, 3438.
/
| 〈 |
|
〉 |