

Chinese Journal of Organic Chemistry >
N-Heterocyclic Carbene-Catalyzed Oxidative Esterification of Aldehydes: Facile Access to α-Acyloxyacetates and Cyanomethyl Esters
Received date: 2018-03-25
Revised date: 2018-06-19
Online published: 2018-07-05
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 20972051, 21476078) and the Science and Technology Commission of Shanghai Municipality (Nos. 12431900900, 12431900902, 08431901800, 08430703900).
An efficient N-heterocyclic carbene-catalyzed oxidative esterification reaction of aldehydes with ethyl bromoacetate or bromoacetonitrile has been explored. This transition metal-free protocol allows access to a wide variety of α-acyloxyacetates and cyanomethyl esters in good to excellent yields under mild reaction condition.
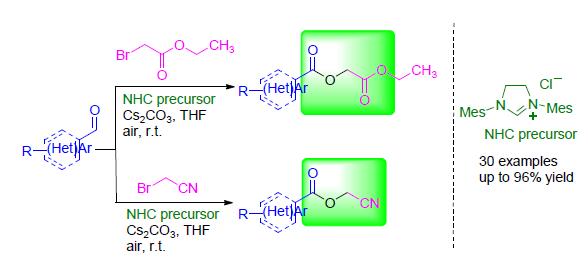
Key words: carbene; aldehydes; esterification; oxidation; organocatalysis
Ju Lei , Ma Chunmei , Tang Mi , Wang Yanhui , Yu Xinhong , Ma Hongmei . N-Heterocyclic Carbene-Catalyzed Oxidative Esterification of Aldehydes: Facile Access to α-Acyloxyacetates and Cyanomethyl Esters[J]. Chinese Journal of Organic Chemistry, 2018 , 38(11) : 3056 -3062 . DOI: 10.6023/cjoc201803040
[1] (a) Barratt, B. J.; Easton, C. J.; Henry, D. J.; Li, I. H.; Radom, L.; Simpson, J. S. J. Am. Chem. Soc. 2004, 126, 13306.
(b) Sarabia, F.; Chammaa, S.; Ruiz, A. S.; Ortiz, L. M.; Herrera, F. L. Curr. Med. Chem. 2004, 11, 1309.
(c) Hamada, Y.; Shioiri, T. Chem. Rev. 2005, 2009, 2009, 5390.
(d) Wang, H.-W.; Liu, Z.-C.; Chen, C.-H.; Lim, T.-S.; Fann, W.; Chao, C.-G.; Yu, J.-Y.; Lee, S.-L.; Chen, C.-H.; Huang, S.-L.; Luh, T.-Y. Chem.-Eur. J. 2009, 15, 5719.
(e) White, J. D.; Jeffrey, S. C. Tetrahedron 2009, 65, 6642.
(f) Nahrwold, M.; Bogner, T.; Eissler, S.; Verma, S.; Sewald, N. Org. Lett. 2010, 12, 1064.
[2] (a) Dräger, G.; Kiss, C.; Kunz, U.; Kirschning, A. Org. Biomol. Chem. 2007, 5, 3657.
(b) Allais, F.; Martinet, S.; Ducrot, P.-H. Synthesis 2009, 3571.
(c) Franz, N.; Menin, L.; Klok, H. A. Eur. J. Org. Chem. 2009, 2009, 5390.
(d) Wang, H.-W.; Liu, Z.-C.; Chen, C.-H.; Lim, T.-S.; Fann, W.; Chao, C.-G.; Yu, J.-Y.; Lee, S.-L.; Chen, C.-H.; Huang, S.-L.; Luh, T.-Y. Chem.-Eur. J. 2009, 15, 5719.
(e) White, J. D.; Jeffrey, S. C. Tetrahedron 2009, 65, 6642.
(f) Nahrwold, M.; Bogner, T.; Eissler, S.; Verma, S.; Sewald, N. Org. Lett. 2010, 12, 1064.
[3] (a) Dumitrescu, L.; Azzouzi-Zriba, K.; Bonnet-Delpon, D.; Crousse, B. Org. Lett. 2011, 13, 692.
(b) Setzer, P.; Forcher, G.; Boeda, F.; Pearson-Long, M. S. M.; Bertus, P. Eur. J. Org. Chem. 2014, 2014, 171.
[4] Katritzky, A. R.; Abdel-Fattah, A. A. A.; Idzik, K. R.; El-Gendy, B. E.-D. M.; Soloducho, J. Tetrahedron 2007, 63, 6477.
[5] (a) Berlozecki, S.; Szymanski, W.; Ostaszewski, R. Tetrahedron 2008, 64, 9780.
(b) Gulevich, A. V.; Shpilevaya, I. V.; Nenajdenko, V. G. Eur. J. Org. Chem. 2009, 2009, 3801.
[6] (a) Shinada, T.; Kawakami, T.; Sakai, H.; Takada, I.; Ohfune, Y. Tetrahedron Lett. 1998, 39, 3757.
(b) Bertelsen, S.; Nielsen, M.; Bachmann, S.; Jørgensen, K. A. Synthesis 2005, 2234.
(c) Vorob'eva, D. V.; Titanyuk, I. D.; Beletskaya, I. P.; Osipov, S. N. Mendeleev. Commun. 2005, 15, 222.
(d) Wang, Z.; Bi, X.; Liang, Y.; Liao, P.; Dong, D. Chem. Commun. 2014, 50, 3976.
[7] (a) Kantchev, E. A. B.; O'Brien, C. J.; Organ, M. G. Angew. Chem. 2007, 119, 2824.
(b) Wuertz, S.; Glorius, F. Acc. Chem. Res. 2008, 41, 1523.
(c) Chass, G. A.; O'Brien, C. J.; Hadei, N.; Kantchev, E. A. B.; Mu, W. H.; Fang, D. C.; Hopkinson, A. C.; Csizmadia, I. G.; Organ, M. G. Chem.-Eur. J. 2009, 15, 4281.
(d) Díez-González, S.; Marion, N.; Nolan, S. P. Chem. Rev. 2009, 109, 3612.
(e) Visbal, R.; Gimeno, M. C. Chem. Soc. Rev. 2014, 43, 3551.
(f) Chirik, P. J. Acc. Chem. Res. 2015, 48, 1687.
[8] (a) Enders, D.; Niemeier, O.; Henseler, A. Chem. Rev. 2007, 107, 5606.
(b) Flanigan, D. M.; Romanov-Michailidis, F.; White, N. A.; Rovis, T. Chem. Rev. 2015, 115, 9307.
(c) Wang, M. H.; Scheidt, K. A. Angew. Chem., Int. Ed. 2016, 55, 14912.
(d) Janssen-Müller, D.; Schlepphorst, C.; Glorius, F. Chem. Soc. Rev. 2017, 46, 4845.
(e) Chen, X.-Y.; Liu, Q.; Chauhan, P.; Enders, D. Angew. Chem., Int. Ed. 2018, 57, 3862.
[9] (a) Enders, D.; Balensiefer, T. Acc. Chem. Res. 2004, 37, 534.
(b) Zeitler, K. Angew. Chem., Int. Ed. 2005, 44, 7506.
(c) Marion, N.; Díez-González, S.; Nolan, S. P. Angew. Chem., Int. Ed. 2007, 46, 2988.
(d) Nair, V.; Vellalath, S.; Babu, B. P. Chem. Soc. Rev. 2008, 37, 2691.
(e) Arduengo Ⅲ, A. J.; Iconaru, L. I. Dalton. Trans. 2009, 6903.
(f) Biju, A. T.; Kuhl, N.; Glorius, F. Acc. Chem. Res. 2011, 44, 1182.
(g) Nair, V.; Menon, R. S.; Biju, A. T.; Sinu, C.; Paul, R. R.; Jose, A.; Sreekumar, V. Chem. Soc. Rev. 2011, 40, 5336.
(h) Rong, Z.-Q.; Zhang, W.; Yang, G.-Q.; You, S.-L. Curr. Org. Chem. 2011, 15, 3077.
(i) Bugaut, X.; Glorius, F. Chem. Soc. Rev. 2012, 41, 3511.
(j) Cohen, D. T.; Scheidt, K. A. Chem. Sci. 2012, 3, 53.
(k) Grossmann, A.; Enders, D. Angew. Chem., Int. Ed. 2012, 51, 314.
(l) De Sarkar, S.; Biswas, A.; Samanta, R. C.; Studer, A. Chem.-Eur. J. 2013, 19, 4664.
(m) Ryan, S. J.; Candish, L.; Lupton, D. W. Chem. Soc. Rev. 2013, 42, 4906.
(n) Mahatthananchai, J.; Bode, J. W. Acc. Chem. Res. 2014, 47, 696.
[10] (a) Kayaki, Y.; Yamamoto, M.; Ikariya, T. Angew. Chem., Int. Ed. 2009, 48, 4194.
(b) Yamashita, K.; Hase, S.; Kayaki, Y.; Ikariya, T. Org. Lett. 2015, 17, 2334.
[11] (a) He, M.; Struble, J. R.; Bode, J. W. J. Am. Chem. Soc. 2006, 128, 8418.
(b) He, M.; Uc, G. J.; Bode, J. W. J. Am. Chem. Soc. 2006, 128, 15088.
(c) Ryan, S. J.; Candish, L.; Lupton, D. W. J. Am. Chem. Soc. 2011, 133, 4694.
(d) Allen, S. E.; Mahatthananchai, J.; Bode, J. W.; Kozlowski, M. C. J. Am. Chem. Soc. 2012, 134, 12098.
(e) Ryan, S. J.; Stasch, A.; Paddon-Row, M. N.; Lupton, D. W. J. Org. Chem. 2012, 77, 1113.
(f) Wang, M.; Huang, Z.; Xu, J.; Chi, Y. R. J. Am. Chem. Soc. 2014, 136, 1214.
(g) Zhu, T.; Zheng, P.; Mou, C.; Yang, S.; Song, B.-A.; Chi, Y. R. Nat. Commun. 2014, 5.
(h) Xu, J.; Hu, S.; Lu, Y.; Dong, Y.; Tang, W.; Lu, T.; Du, D. Adv. Synth. Catal. 2015, 357, 923.
[12] (a) Reynolds, N. T.; Read de Alaniz, J.; Rovis, T. J. Am. Chem. Soc. 2004, 126, 9518.
(b) Chan, A.; Scheidt, K. A. Org. Lett. 2005, 7, 905.
(c) Sohn, S. S.; Bode, J. W. Angew. Chem. 2006, 118, 6167.
(d) Bode, J. W.; Sohn, S. S. J. Am. Chem. Soc. 2007, 129, 13798.
(e) Li, G.-Q.; Li, Y.; Dai, L.-X.; You, S.-L. Org. Lett. 2007, 9, 3519.
(f) Kaeobamrung, J.; Mahatthananchai, J.; Zheng, P.; Bode, J. W. J. Am. Chem. Soc. 2010, 132, 8810.
[13] DiRocco, D. A.; Rovis, T. J. Am. Chem. Soc. 2012, 134, 8094.
[14] (a) Candish, L.; Lupton, D. W. Chem. Sci. 2012, 3, 380.
(b) Wu, Z.; Wang, X.; Li, F.; Wu, J.; Wang, J. Org. Lett. 2015, 17, 3588.
[15] (a) Cardinal-David, B.; Raup, D. E.; Scheidt, K. A. J. Am. Chem. Soc. 2010, 132, 5345.
(b) Raup, D. E.; Cardinal-David, B.; Holte, D.; Scheidt, K. A. Nat. Chem. 2010, 2, 766.
(c) Zhao, X.; DiRocco, D. A.; Rovis, T. J. Am. Chem. Soc. 2011, 133, 12466.
(d) Cohen, D. T.; Scheidt, K. A. Chem. Sci. 2012, 3, 53.
(e) Dugal-Tessier, J.; O'Bryan, E. A.; Schroeder, T. B.; Cohen, D. T.; Scheidt, K. A. Angew. Chem., Int. Ed. 2012, 51, 4963.
(f) Zhang, Y.; Lu, Y.; Tang, W.; Lu, T.; Du, D. Org. Biomol. Chem. 2014, 12, 3009.
(g) Lin, Y.; Yang, L.; Deng, Y.; Zhong, G. Chem. Commun. 2015, 51, 8330.
(h) Xu, J.; Chen, X.; Wang, M.; Zheng, P.; Song, B.-A.; Chi, Y. R. Angew. Chem. 2015, 127, 5250.
[16] (a) Maki, B. E.; Scheidt, K. A. Org. Lett. 2008, 10, 4331.
(b) De Sarkar, S.; Grimme, S.; Studer, A. J. Am. Chem. Soc. 2010, 132, 1190.
(c) Maji, B.; Vedachalan, S.; Ge, X.; Cai, S.; Liu, X. W. J. Org. Chem. 2011, 76, 3016.
(d) Delany, E. G.; Fagan, C. L.; Gundala, S.; Mari, A.; Broja, T.; Zeitler, K.; Connon, S. J. Chem. Commun. 2013, 49, 6510.
(e) Berry, M. T.; Castrejon, D.; Hein, J. E. Org. Lett. 2014, 16, 3676.
(f) Samanta, R. C.; Studer, A. Org. Chem. Front. 2014, 1, 936.
[17] Knappke, C. E. I.; Imami, A.; Jacobivon Wangelin, A. ChemCatChem 2012, 4, 937.
[18] (a) De Sarkar, S.; Studer, A. Angew. Chem., Int. Ed. 2010, 49, 9266.
(b) Rose, C. A.; Zeitler, K. Org. Lett. 2010, 12, 4552.
(c) Park, J. H.; Bhilare, S. V.; Youn, S. W. Org. Lett. 2011, 13, 2228.
(d) Sun, Z.-X.; Cheng, Y. Org. Biomol. Chem. 2012, 10, 4088.
(e) Youn, S. W.; Song, H. S.; Park, J. H. Org. Biomol. Chem. 2014, 12, 2388.
(f) Youn, S. W.; Song, H. S.; Park, J. H. Org. Lett. 2014, 16, 1028.
[19] Rosa, J. N.; Reddy, R. S.; Candeias, N. R.; Cal, P. M.; Gois, P. M. Org. Lett. 2010, 12, 2686.
[20] Li, Y.; Du, W.; Deng, W.-P. Tetrahedron 2012, 68, 3611.
[21] Xin, Y.-C.; Shi, S.-H.; Xie, D.-D.; Hui, X.-P.; Xu, P.-F. Eur. J. Org. Chem. 2011, 2011, 6527.
[22] Ramanjaneyulu, B. T.; Reddy, V.; Arde, P.; Mahesh, S.; Anand, R. V. Chem.-Asian J. 2013, 8, 1489.
[23] Zhang, Y.; Du, Y.; Huang, Z.; Xu, J.; Wu, X.; Wang, Y.; Wang, M.; Yang, S.; Webster, R. D.; Chi, Y. R. J. Am. Chem. Soc. 2015, 137, 2416.
[24] Li, B.-S.; Wang, Y.; Jin, Z.; Zheng, P.; Ganguly, R.; Chi, Y. R. Nat. Commun. 2015, 6, 6207.
/
| 〈 |
|
〉 |