

Chinese Journal of Organic Chemistry >
Biosynthesis of Fungal Triterpenoids and Steroids
Received date: 2018-06-20
Revised date: 2018-07-06
Online published: 2018-07-16
Supported by
Project supported by the National Natural Science Foundation of China (No. 31670036).
Triterpenoids and steroids are one of the largest classes of natural products composed of six isoprene units, with various chemical structures as well as wide range of biological activities. Fungi serves as important sources for triterpenoids and steroids. However, compared with plants, the types of triterpenoid skeletons discovered in fungi are much fewer, suggesting that there is a large research space. Genome mining has become an important method of discovering novel natural products in the post-genomic era, which uses the genes with similar functional to identify the target genes with new functions. With the rapid development of high-throughput sequencing and biological information technology, biosynthetic pathways of some triterpenoids and steroids with important biological activities have been elucidated in recent years, which build the foundations for the discovery of new triterpenoids or steroids from fungi via genome mining. The recent advances in the biosynthesis of fungal triterpenoids and steroids are mainly introduced.
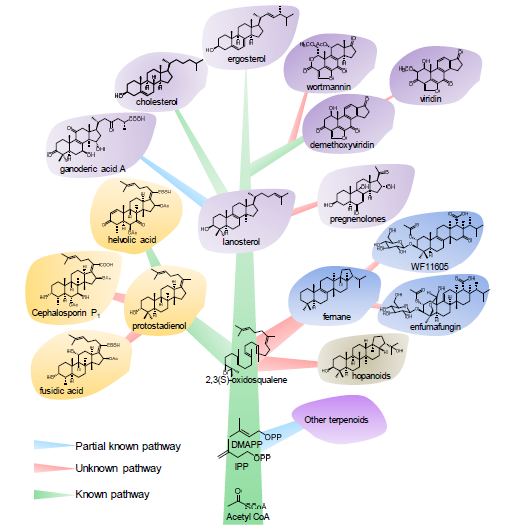
Key words: triterpenoids; steroids; fungi; biosynthesis; genome mining
Gao Yaohui , Wang Gaoqian , Huang Huiyun , Gao Hao , Yao Xinsheng , Hu Dan . Biosynthesis of Fungal Triterpenoids and Steroids[J]. Chinese Journal of Organic Chemistry, 2018 , 38(9) : 2335 -2347 . DOI: 10.6023/cjoc201806033
[1] Hill, R. A.; Connolly, J. D. Nat. Prod. Rep. 2017, 34, 90.
[2] Thimmappa, R.; Geisler, K.; Louveau, T.; O'Maille, P.; Osbourn, A. Annu. Rev. Plant Biol. 2014, 65, 225.
[3] Schaller, H. In Comprehensive Natural Products Ⅱ, Elsevier, Oxford, 2010, pp. 755~787.
[4] Arora, A.; Raghuraman, H.; Chattopadhyay, A. Biochem. Biophys. Res. Commun. 2004, 318, 920.
[5] Beck, J. G.; Mathieu, D.; Loudet, C.; Buchoux, S.; Dufourc, E. J. FASEB J. 2007, 21, 1714.
[6] Frye, C. A. Minerva Ginecol. 2009, 61, 541.
[7] Funder, J. W.; Krozowski, Z.; Myles, K.; Sato, A.; Sheppard, K. E.; Young, M. Recent Prog. Horm. Res. 1997, 52, 247~260; discussion 261~262.
[8] Schaaf, M. J.; Cidlowski, J. A. J. Steroid Biochem. Mol. Biol. 2002, 83, 37.
[9] Sun, M.; Ye, Y.; Xiao, L.; Duan, X.; Zhang Y.; Zhang, H. Int. J. Mol. Med. 2017, 39, 507.
[10] Ali-Seyed, M.; Jantan, I.; Vijayaraghavan, K.; Bukhari, S. N. Chem. Biol. Drug Des. 2016, 87, 517.
[11] Zhang, H. F.; Semenza, G. L. Proc. Natl. Acad. Sci. U. S. A. 2009, 106, E27.
[12] Ming, L. J.; Yin, A. C. Nat. Prod. Commun. 2013, 8, 415.
[13] Bishop, K. S.; Kao, C. H. J.; Xu, Y. Y.; Glucina, M. P.; Paterson R. R. M.; Ferguson, L. R. Phytochemistry 2015, 114, 56.
[14] Tanret, C. C. R. Seances Acad. Sci. 1889, 108, 98.
[15] Rios, J. L.; Andujar, I.; Recio, M. C.; Giner, R. M. J. Nat. Prod. 2012, 75, 2016.
[16] Xu, J. W.; Zhao, W.; Zhong, J. J. Appl. Microbiol. Biotechnol. 2010, 87, 457.
[17] Weete, J. D.; Abril, M.; Blackwell, M. PLoS One 2010, 5, e10899.
[18] Hirotani, M.; Hirotani, S.; Takayanagi, H.; Yoshikawa, T. Tetrahedron Lett. 1999, 40, 329.
[19] Kikuchi, T.; Masumoto, Y.; In, Y.; Tomoo, K.; Yamada, T.; Tanaka, R. Eur. J. Org. Chem. 2015, 4645.
[20] Kikuchi, T.; Maekawa, Y.; Tomio, A.; Masumoto, Y.; Yamamoto, T.; In, Y.; Yamada, T.; Tanaka, R. Steroids 2016, 115, 9.
[21] Kikuchi, T.; Horii, Y.; Maekawa, Y.; Masumoto, Y.; In, Y.; Tomoo, K.; Sato, H.; Yamano, A.; Yamada, T.; Tanaka, R. J. Org. Chem. 2017, 82, 10611.
[22] Hu, Z.; Wu, Y.; Xie, S.; Sun, W.; Guo, Y.; Li, X. N.; Liu, J.; Li, H.; Wang, J.; Luo, Z.; Xue, Y.; Zhang, Y. Org. Lett. 2017, 19, 258.
[23] Han, J. J.; Bao, L.; Tao, Q. Q.; Yao, Y. J.; Liu, X. Z.; Yin, W. B.; Liu, H. W. Org. Lett. 2015, 17, 2538.
[24] Luo, Q.; Tu, Z. C.; Yang, Z. L.; Cheng, Y. X. Fitoterapia 2018, 125, 273.
[25] Weete, J. D.; Fuller, M. S.; Huang, M. Q.; Gandhi, S. Exp. Mycol. 1989, 13, 183.
[26] Muchembled, J.; Alh, S.; Grandmouginferjani, A.; Sancholle, M. Can. J. Bot. 2000, 78, 1288.
[27] Chepkirui, C.; Sum, W. C.; Cheng, T.; Matasyoh, J. C.; Decock C.; Stadler, M. Molecules 2018, 23.
[28] Elsebai, M. F.; Kehraus, S.; Konig, G. M. Steroids 2013, 78, 880.
[29] Zhao, Q.; Wang, G. Q.; Chen, G. D.; Hu, D.; Li, X. X.; Guo, L. D.; Li, Y.; Yao, X. S.; Gao, H. Steroids 2015, 102, 101.
[30] Blight, M. M.; Grove, J. F. J. Chem. Soc., Perkin Trans. 1, 1986, 7, 1317.
[31] Andersson, P. F.; Bengtsson, S.; Cleary, M.; Stenlid, J.; Broberg, A. Phytochemistry 2013, 86, 195.
[32] Ding, H. E.; Yang, Z. D.; Sheng, L.; Zhou, S. Y.; Li, S.; Yao, X. J.; Zhi, K. K.; Wang, Y. G.; Zhang, F. Tetrahedron Lett. 2015, 56, 6754.
[33] Jimeno, A.; Bauman, J. E.; Weissman, C.; Adkins, D.; Schnadig, I.; Beauregard, P.; Bowles, D. W.; Spira, A.; Levy, B.; Seetharamu, N. Oral Oncol. 2015, 51, 383.
[34] Zhao, Q.; Chen, G. D.; Feng, X. L.; Yu, Y.; He, R. R.; Li, X. X.; Huang, Y.; Zhou, W. X.; Guo, L. D.; Zheng, Y. Z. J. Nat. Prod. 2015, 78, 1221.
[35] Cao, S.; Ross, L.; Tamayo, G.; Clardy, J. Org. Lett. 2010, 12, 4661.
[36] Lv, J.-M.; Hu, D.; Gao, H.; Kushiro, T.; Awakawa, T.; Chen, G.-D.; Wang, C.-X.; Abe, I.; Yao, X.-S. Nat. Commun. 2017, 8, 1644.
[37] Deyrup, S. T.; Gloer, J. B.; O'Donnell, K.; Wicklow, D. T. J. Nat. Prod. 2007, 70, 378.
[38] Joshi, B. K.; Gloer, J. B.; Wicklow, D. T. J. Nat. Prod. 2002, 65, 1734.
[39] Schwartz, R. E.; Smith, S. K.; Onishi, J. C.; Meinz, M.; Kurtz, M.; Giacobbe, R. A.; Wilson, K. E.; Liesch, J.; Zink, D.; Horn, W.; Morris, S.; Cabello, A.; Vicente, F. J. Am. Chem. Soc. 2000, 122, 4882.
[40] Shigematsu, N.; Tsujii, E.; Kayakiri, N.; Takase, S.; Tanaka, H.; Tada, T. J. Antibiot. (Tokyo) 1992, 45, 704.
[41] Kumla, D.; Shine, A. T.; Buttachon, S.; Dethoup, T.; Gales, L.; Pereira, J. A.; Inácio, A.; Costa, P. M.; Lee, M.; Sekeroglu, N. Mar. Drugs 2017, 15, 375.
[42] Kristan, K.; Rizner, T. L. J. Steroid Biochem. 2012, 129, 79.
[43] Kalb, V. F.; Woods, C. W.; Turi, T. G.; Dey, C. R.; Sutter, T. R.; Loper, J. C. DNA 1987, 6, 529.
[44] Lepesheva, G. I.; Waterman, M. R. Mol. Cell. Endocrinol. 2004, 215, 165.
[45] Ghannoum, M. A.; Rice, L. B. Clin. Microbiol. Rev. 1999, 12, 501.
[46] Lai, M. H.; Bard, M.; Pierson, C. A.; Alexander, J. F.; Goebl, M.; Carter, G. T.; Kirsch, D. R. Gene 1994, 140, 41.
[47] Akins, R. A.; Sobel, J. D. Med. Mycol. 2017, 347.
[48] Blosser, S. J.; Merriman, B.; Grahl, N.; Chung, D.; Cramer, R. A. Microbiol. 2014, 160, 2492.
[49] Aaron, K. E.; Pierson, C. A.; Lees, N. D.; Bard, M. Fems Yeast Res. 2001, 1, 93.
[50] Daum, G.; Lees, N. D.; Bard, M.; Dickson, R. Yeast 1998, 14, 1471.
[51] Mo, C.; Valachovic, M.; Randall, S. K. J.; Nickels, T.; Bard, M. P. Natl. Acad. Sci. U. S. A. 2002, 99, 9739.
[52] Mo, C. Q.; Bard, M. J. Lipid Res. 2005, 46, 1991.
[53] Keon, J. P.; James, C. S.; Court, S.; Baden-Daintree, C.; Bailey, A. M.; Burden, R. S.; Bard, M.; Hargreaves, J. A. Curr. Genet. 1994, 25, 531.
[54] Taton, M.; Husselstein, T.; Benveniste, P.; Rahier, A. Biochemistry 2000, 39, 701.
[55] Kelly, S. L.; Lamb, D. C.; Corran, A. J.; Baldwin, B. C.; Parks, L. W.; Kelly, D. E. FEBS Lett. 1995, 377, 217.
[56] Zweytick, D.; Hrastnik, C.; Kohlwein, S. D.; Daum, G. FEBS Lett. 2000, 470, 83.
[57] Wang, F.-Q.; Zhao, Y.; Dai, M.; Liu, J.; Zheng, G.-Z.; Ren, Z.-H.; He, J.-G. FEMS Microbiol. Lett. 2008, 287, 91.
[58] Long, N.; Xu, X.; Zeng, Q.; Sang, H.; Lu, L. Appl. Environ. Microbiol. 2017, 83.
[59] Barrett-Bee, K.; Dixon, G. Acta Biochim. Pol. 1995, 42, 465.
[60] Ragsdale, N. N. Biochim. Biophys. Acta 1975, 380, 81.
[61] Hajjaj, H; Mace, C.; Roberts, M.; Niederberger, P.; Fay, L. B. Appl. Environ. Microbiol. 2005, 71, 3653.
[62] Gao, J. J.; Min, B. S.; Ahn, E. M.; Nakamura, N.; Lee, H. K.; Hattori, M. Chem. Pharm. Bull. 2002, 50, 837.
[63] Shi, L. A.; Ren, A.; Mu, D. S.; Zhao, M. W. Appl. Microbiol. Biotechnol. 2010, 88, 1243.
[64] Zhao, M.-W.; Liang, W.-Q.; Zhang, D.-B.; Wang, N.; Wang, C.-G.; Pan, Y.-J. J. Microbiol. Biotechnol. 2007, 17, 1106.
[65] Shang, C.-H.; Zhu, F.; Li, N.; Ou-yang, X.; Shi, L.; Zhao, M.-W.; Li, Y.-X. Biosci. Biotechnol. Biochem. 2008, 72, 1333.
[66] Shi, L.; Qin, L.; Xu, Y. J.; Ren, A.; Fang, X.; Mu, D. S.; Tan, Q.; Zhao, M. W. Mol. Biol. Rep. 2012, 39, 6149.
[67] Ding, Y.-X.; Ou-yang, X.; Shang, C.-H.; Ren, A.; Shi, L.; Li, Y.-X.; Zhao, M.-W. Biosci. Biotechnol. Biochem. 2008, 72, 1571.
[68] Shang, C.-H.; Shi, L.; Ren, A.; Qin, L.; Zhao, M.-W. Biosci. Biotechnol. Biochem. 2010, 74, 974.
[69] Chen, S.; Xu, J.; Liu, C.; Zhu, Y.; Nelson, D. R.; Zhou, S.; Li, C.; Wang, L.; Guo, X.; Sun, Y.; Luo, H.; Li, Y.; Song, J.; Henrissat, B.; Levasseur, A.; Qian, J.; Li, J.; Luo, X.; Shi, L.; He, L.; Xiang, L.; Xu, X.; Niu, Y.; Li, Q.; Han, M. V.; Yan, H.; Zhang, J.; Chen, H.; Lv, A.; Wang, Z.; Liu, M.; Schwartz, D. C.; Sun, C. Nat. Commun. 2012, 3, 913.
[70] Wang, W.-F.; Xiao, H.; Zhong, J.-J. Biotechnol. Bioeng. 2018, 115, 1842.
[71] Von Daehne, W.; Godtfredsen, W. O.; Rasmussen, P. R. Adv. Appl. Microbiol. 1979, 25, 95.
[72] Caspi, E.; Mulheirn, L. J. J. Am. Chem. Soc. 1970, 92, 404.
[73] Chain, E.; Florey, H. W.; Jennings, M. A.; Willliams, T. I. Brit. J. Exp. Pathol. 1943, 24, 108.
[74] Godtfredsen, W. O.; Jahnsen, S.; Lorck, H.; Roholt, K.; Tybring, L. Nature 1962, 193, 987.
[75] Burton, H. S.; Abraham, E. P. Biochem. J. 1951, 50, 168.
[76] Mitsuguchi, H.; Seshime, Y.; Fujii, I.; Shibuya, M.; Ebizuka, Y.; Kushiro, T. J. Am. Chem. Soc. 2009, 131, 6402.
[77] Simpson, T. J.; Lunnon, M. W.; MacMillan, J. J. Chem. Soc., Perkin Trans. 11979, 931.
[78] Hanson, J. R.; Wadsworth, H. J. B. J. Chem. Soc., Chem. Commun. 1979, 360.
[79] Grove, J. F. J. Chem. Soc. 1969, C, 549.
[80] Golder, W. S.; Watson, T. R. J. Chem. Soc., Perkin Trans. 11980, 422.
[81] Hanson, J. R.; O'Leary, M. A.; Wadsworth, H. J. J. Chem. Soc., Perkin Trans. 11983, 871.
[82] Alcazar-Fuoli, L.; Mellado, E.; Garcia-Effron, G.; Lopez, J. F.; Grimalt, J. O.; Cuenca-Estrella, J. M.; Rodriguez-Tudela, J. L. Steroids 2008, 73, 339.
[83] Wang, G.-Q.; Chen, G.-D.; Qin, S.-Y.; Hu, D.; Awakawa, T.; Li, S.-Y.; Lv, J.-M.; Wang, C.-X.; Yao, X.-S.; Abe, I.; Gao, H. Nat. Commun. 2018, 9, 1838.
/
| 〈 |
|
〉 |