

Chinese Journal of Organic Chemistry >
Synthesis and Fungicidal Evaluation of Novel β-Carboline-Benzimidazole and β-Carboline-Benzothiazole Hybrids
Received date: 2018-05-29
Revised date: 2018-07-07
Online published: 2018-08-22
Supported by
Project supported by the Scientific Research Innovation Project in Xinjiang Uygur Autonomous Region (No. XJGRI2017045), the National Students Innovation and Entrepreneuship Training Program (No. 201810759055), the Program for Changjiang Scholars and Innovative Research Team in University (No. IRT15R46), and the Yangtze River Scholar Research Project of Shihezi University (No. CJXZ201601).
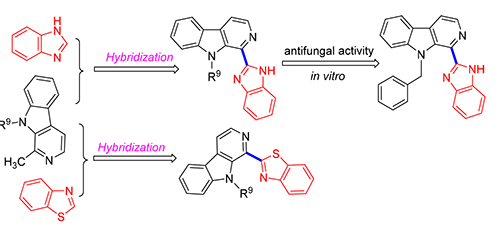
In order to discover novel compounds with biological activities, new molecular hybrids combining benzimidazole or its bioisostere benzothiazole with β-carboline were synthesized. The benzimidazole or benzothiazole scaffold was linked at position-1 with β-carboline which was further characterized by 1H NMR, 13C NMR and HRMS. All of the target compounds were evaluated in vitro for their antifungal activity against Rhizoctorzia solani, Fusarium oxysporum, Botrytis cinerea Pers., sunflower sclerotinia rot and rape sclerotinia rot by mycelia growth inhibition assay at 50 μg·mL-1. The preliminary results showed that most compounds exhibit mild inhibiting effect against all the tested strains. Among them, 1-(1H-benzo[d]-imida-zol-2-yl)-9-ethyl-β-carboline (4a), 1-(1H-benzo[d]imidazol-2-yl)-9-benzyl-β-carboline (4c) and 1-(1H-benzo[d]imidazol-2-yl)-9-(3-chlorobenzyl)-β-carboline (4e) showed satisfactory antifungal activity against sunflower sclerotinia rot, 1-(1H-benzo[d]-imidazol-2-yl)-9-ethyl-β-carboline (4a), 1-(1H-benzo[d]imidazol-2-yl)-9-n-butyl-β-carboline (4b), 1-(1H-benzo[d]imidazol-2-yl)-9-benzyl-β-carboline (4c), 1-(1H-benzo[d]imidazol-2-yl)-9-((perfluorophenyl)methyl)-β-carboline (4f), 1-(benzo[d]-thiazol-2-yl)-9-(3-chlorobenzyl)-β-carboline (5e) and 1-(benzo[d]thiazol-2-yl)-9-((perfluorophenyl)methyl)-β-carboline (5f) displayed excellent fungicidal activity against rape sclerotinia rot. Specifically, compound 4c exhibited broad-spectrum fungicidal activity against most of the tested fungi.
Key words: β-carboline; benzimidazole; benzothiazole; antifungal activity
Huo Xinyu , Li Wenbin , Zhang Boya , Chen Xiaofei , Zhou Yueting , Zhang Jie , Han Xiaoqiang , Dai Bin . Synthesis and Fungicidal Evaluation of Novel β-Carboline-Benzimidazole and β-Carboline-Benzothiazole Hybrids[J]. Chinese Journal of Organic Chemistry, 2018 , 38(12) : 3356 -3362 . DOI: 10.6023/cjoc201805053
[1] Qiu, D.-W.; Dong, Y.-J.; Zhang, Y.; Li, S.-P.; Shi, F.-C. Mol Plant Microbe Interact. 2017, 30, 355.
[2] Xu, W.-M.; He, J.; He, M.; Han, F.-F.; Chen, X.-H.; Pan, Z.-X.; Wang, J.; Tong, M.-G. Molecules 2011, 16, 9129.
[3] Pavela, R.; Vrchotová, N. Ind. Crops Prod. 2013, 43, 33.
[4] Meester, C. D. Mutat. Res. 1995, 339, 139.
[5] Michael, C.; Robert, W. W.; Fil, G.; James, M. C.; Steven, A. B.; Kenner, C. R.; Jacqueline, N. C.; Steven, M. P.; Phil, S. J. Med. Chem. 1982, 25, 1081.
[6] Bournine, L.; Bensalem, S.; Fatmi, S.; Bedjou, F.; Mathieu, V.; Iguer-Ouada, M.; Kiss, R.; Duez, P. Eur. J. Integr. Med. 2017, 9, 91.
[7] Asgarpanah, J.; Ramezanloo, F. Afr. J. Pharm. Pharmacol. 2012, 6, 1573.
[8] Srivastava, S. K.; Agarwal, A.; Chauhan, P. M. S.; Agarwal, S. K.; Bhaduri, A. P.; Singh, S. N.; Fatima, N.; Chatterjee, R. K. Bioorg. Med. Chem. 1999, 7, 1223.
[9] Wang, Y.-H.; Tang, J.-G.; Wang, R.-R.; Yang, L.-M.; Dong, Z.-J.; Du, L.; Shen, X.; Liu, J.-K.; Zheng, Y.-T. Biochem. Biophys. Res. Commun. 2007, 355, 1091.
[10] Zhang, Z.-J.; Zhang, J.-J.; Jiang, Z.-Y.; Zhong, G.-H. Molecules 2017, 22, 1811.
[11] Nenaah, G. J. Stored Prod. Res. 2011, 47, 255.
[12] Abbasipour, H.; Mahmoudvand, M.; Rastegar, F.; Basij, M. Bull. Insectol. 2010, 63, 259.
[13] Song, H.-J.; Liu, Y.-X.; Liu, Y.-X.; Wang, Q.-M. J. Agric. Food Chem. 2014, 62, 1010.
[14] Huang, Y.-Q.; Liu, Y.-X.; Liu, Y.-X.; Song, H.-J.; Wang, Q.-M. Bioorg. Med. Chem. 2016, 24, 462.
[15] Li, Z.-B.; Chen, S.-H.; Zhu, S.-W.; Luo, J.-J.; Zhang, Y.-M.; Weng, Q.-F. Molecules 2015, 20, 13941.
[16] Huo, X.-Y.; Guo, L.; Wei, Y.-T.; Zhang, J.; Han, X.-Q. Agrochemicals 2018, 57, 3(in Chinese). (霍新玉, 郭亮, 韦玥婷, 张洁, 韩小强, 农药, 2018, 57, 3.)
[17] Patil, S. A.; Patil, S. A.; Patil, R. Chem. Biol. Drug Des. 2017, 89, 639.
[18] El-Gohary, N. S.; Shaaban, M. I. Eur. J. Med. Chem. 2017, 131, 255.
[19] Alasmary, F. A. S.; Snelling, A. M.; Zain, M. E.; Alafeefy, A. M.; Awaad, A. S.; Karodia, N. Molecules 2015, 20, 15206.
[20] Gray, L. E.; Ostby, J.; Linder, R.; Goldman, J.; Rehnberg, G.; Cooper, R. Fundam. Appl. Toxicol. 1990, 15, 281.
[21] Feng, J.-Y.; Hu, Y.-X.; Grant, E.; Lu, X.-N. Food Chem. 2018, 239, 816.
[22] Zhao, S.-Z.; Zhao, L.-Y.; Zhang, X.-Q.; Liu, C.-C.; Hao, C.-Z.; Xie, H.-L.; Sun, B.; Zhao, D.-M.; Cheng, M.-S. Eur. J. Med. Chem. 2016, 123, 514.
[23] Chugunova, E.; Boga, C.; Sazykin, I.; Cino, S.; Micheletti, G.; Mazzanti, A.; Sazykina, M.; Burilov, A.; Khmelevtsova, L.; Kostina, N. Eur. J. Med. Chem. 2015, 93, 349.
[24] Tomlin, C. E. The Pesticide Manual, 11th ed., The British Crop Protection Council, Surrey, 1997, p. 463.
[25] Smith, A. E. J. Agric. Food Chem. 1985, 33, 483.
[26] Guo, L.; Fan, W.-X.; Chen, W.; Ma, Q.; Cao, R.-H. J. Chin. Pharm. Sci. 2015, 24, 801.
[27] (a) Guo, L.; Fan, W.-X.; Chen, X.-M.; Ma, Q.; Cao, R.-H. Chin. J. Org. Chem. 2013, 33, 332(in Chinese). (郭亮, 范文玺, 陈雪梅, 马芹, 曹日晖, 有机化学, 2013, 33, 332.)
(b) Guo, L.; Xie, J.-W.; Fan, W.-X.; Chen, W.; Dai, B.; Ma, Q. Chin. J. Org. Chem. 2017, 37, 1741(in Chinese). (郭亮, 谢建伟, 范文玺, 陈伟, 代斌, 马芹, 有机化学, 2017, 37, 1741.)
[28] Chen, W.; Zhang, G.-X.; Guo, L.; Fan, W.-X.; Ma, Q.; Zhang, X.-D.; Du, R.-L.; Cao, R.-H. Eur. J. Med. Chem. 2016, 124, 249.
[29] Yuan, X.-Y.; Zhang, L.; Han, X.-Q.; Zhou, Z.-Y.; Du, S.-J.; Wan, C.; Yang, D.-Y.; Qin, Z.-H. Chin. J. Org. Chem. 2014, 34, 170(in Chinese). (袁小勇, 张鹭, 韩小强, 周子原, 杜士杰, 万川, 杨冬燕, 覃兆海, 有机化学, 2014, 34, 170.)
[30] Pesticides Guidelines for Laboratory Bioactivity Tests:Part 2:Petri Plate Test for Determining Fungicide Inhibition of Mycelial Growth:NY/T1156.2-2006, China Agriculture Press, Beijing, 2006(in Chinese). (农药室内生物测定试验准则杀菌剂第2部分:抑制病原真菌菌丝生长试验平皿法:NY/T1156.2-2006. 中国农业出版社, 北京, 2006.)
/
| 〈 |
|
〉 |