

Chinese Journal of Organic Chemistry >
Progress in Heterocycles-Based Asymmetric Vinylogous Mannich Reactions and Applications to the Synthesis of Alkaloids
Received date: 2018-06-03
Revised date: 2018-08-22
Online published: 2018-08-22
Supported by
Project supported by the National Key R&D Program of China (No. 2017YFA0207302), the National Natural Science Foundation of China (Nos. 21332007, 21472153), the Program for Changjiang Scholars and Innovative Research Team in University (PCSIRT) of Ministry of Education, the Chinese Universities Scientific Fund (Nos. 20720170092, 20720180024) and the Natural Science Foundation of Fujian Province of China (No. 2017J01021).
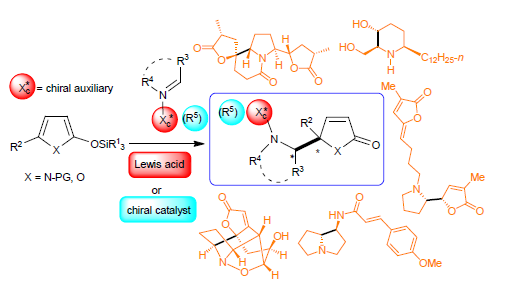
Heterocycles (α,β/β,γ-unsaturated-γ-lactones, α,β-unsaturated-γ-lactams)-based vinylogous Mannich reactions (VMR) constitute a class of effective C-C bond formation approach to install vicinal aminol-containing α,β-unsaturated-lactones and vicinal diamine-containing α,β-unsaturated-γ-lactams. Possessing multiple functionalities, the latters are versatile building blocks for the synthesis of O-heterocycles, N-heterocycles and the synthesis of alkaloids. The progresses of the asymmetric vinylogous Mannich reactions of silyloxy pyrroles and silyloxy furans from 2011 to mid-2018 are summarized. The methods are organized according to chiral auxiliary-induced asymmetric VMRs, asymmetric VMRs catalyzed by metal-chiral ligand complex or organocatalyst, and the applications of the aymmetric VMRs to the syntheses of complex alkaloids. Some limitations of the developed heterocycles-based VMRs are also briefly discussed.
Ye Jianliang , Huang Peiqiang . Progress in Heterocycles-Based Asymmetric Vinylogous Mannich Reactions and Applications to the Synthesis of Alkaloids[J]. Chinese Journal of Organic Chemistry, 2018 , 38(9) : 2215 -2230 . DOI: 10.6023/cjoc201806005
[1] Liu, Y.; Wu, Q.; Yin, D.; Li, D. Chin. J. Org. Chem. 2016, 36, 927(in Chinese). (刘玉婷, 吴倩倩, 尹大伟, 李荻扬, 有机化学, 2016, 36, 927.)
[2] (a) Wehlauch, R.; Gademann, K. Asian J. Org. Chem. 2017, 6, 1146.
(b) Martin, S. F. Adv. Heterocycl. Chem. 2013, 110, 73.
[3] (a) Zhang, Q.; Liu, X.; Feng, X. Curr. Org. Synth. 2013, 10, 764.
(b) Casiraghi, G.; Battistini, L.; Curti, C.; Rassu, G.; Zanardi, F. Chem. Rev. 2011, 111, 3076.
(c) Casiraghi, G.; Zanardi, F.; Battistini, L.; Rassu, G. Synlett 2009, 1525.
(d) Martin, S. F. Acc. Chem. Res. 2002, 35, 895.
(e) Bur, S. K.; Martin, S. F. Tetrahedron 2001, 57, 3221.
[4] Sartori, A.; Dell'Amico, L.; Curti, C.; Battistini, L.; Pelosi, G.; Rassu, G.; Casiraghi, G.; Zanardi, F. Adv. Synth. Catal. 2011, 353, 3278.
[5] Sartori, A.; Dell'Amico, L.; Battistini, L.; Curti, C.; Rivara, S.; Pala, D.; Kerry, P. S.; Pelosi, G.; Casiraghi, G.; Rassu, G.; Zanardi, F. Org. Biomol. Chem. 2014, 12, 1561.
[6] Robak, M. T.; Herbage, M. A.; Ellman, J. A. Chem. Rev. 2010, 110, 3600.
[7] Ruan, S.-T.; Luo, J.-M.; Du, Y.; Huang, P.-Q. Org. Lett. 2011, 13, 4938.
[8] Bur, S. K.; Martin, S. F. Org. Lett. 2000, 2, 3445.
[9] Harding, K. E.; Southard, J. M. Tetrahedron:Asymmetry 2005, 16, 1845.
[10] Guo, L.-D.; Liang, P.; Zheng, J.-F.; Huang, P.-Q. Eur. J. Org. Chem. 2013, 2230.
[11] Liu, R.-C.; Wei, J.-H.; Wei, B.-G.; Lin, G.-Q. Tetrahedron:Asymmetry 2008, 19, 2731.
[12] Altenbach, H.-J.; Himmeldirk, K. Tetrahedron:Asymmetry 1995, 6, 1077.
[13] Banba, Y.; Abe, C.; Nemoto, H.; Kato, A.; Adachi, I.; Takahata, H. Tetrahedron:Asymmetry 2001, 12, 817.
[14] Nadin, A.; Sánchez López, J. M.; Neduvelil, J. G.; Thomas, S. R. Tetrahedron 2001, 57, 1861.
[15] Rao, V. U. B.; Jadhav, A. P.; Garad, D.; Singh, R. P. Org. Lett. 2014, 16, 648.
[16] Shi, Y.-H.; Wang, Z.; Shi, Y.; Deng, W.-P. Tetrahedron 2012, 68, 3649.
[17] Liu, L.-J.; Chen, L.-J.; Li, P.; Li, X.-B.; Liu, J.-T. J. Org. Chem. 2011, 76, 4675.
[18] Liu, L.-J.; Liu, J.-T. Tetrahedron 2014, 70, 1236.
[19] Yu, J.; Miao, Z.; Chen, R. Org. Biomol. Chem. 2011, 9, 1756.
[20] Tamura, O.; Takeda, K.; Mita, N.; Sakamoto, M.; Okamoto, I.; Morita, N.; Ishibashi, H. Org. Biomol. Chem. 2011, 9, 7411.
[21] Degiorgis, F.; Lombardo, M.; Trombini, C. Tetrahedron 1997, 53, 11721.
[22] Garner, P.; Park, J. M. J. Org. Chem. 1990, 55, 3772.
[23] Yuan, Z.-L.; Jiang, J.-J.; Shi, M. Tetrahedron 2009, 65, 6001.
[24] Zhao, Q.-Y.; Yuan, Z.-L.; Shi, M. Tetrahedron:Asymmetry 2010, 21, 943.
[25] Zhao, Q.-Y.; Yuan, Z.-L.; Shi, M. Adv. Synth. Catal. 2011, 353, 637.
[26] Zheng, L.-S.; Li, L.; Yang, K.-F.; Zheng, Z.-J.; Xiao, X.-Q.; Xu, L.-W. Tetrahedron 2013, 69, 8777.
[27] Hayashi, M.; Sano, M.; Funahashi, Y.; Nakamura, S. Angew. Chem., Int. Ed. 2013, 52, 5557.
[28] (a) Carswell, E. L.; Snapper, M. L.; Hoveyda, A. H. Angew. Chem., Int. Ed. 2006, 45, 7230.
(b) Mandai, H.; Mandai, K.; Snapper, M. L.; Hoveyda, A. H. J. Am. Chem. Soc. 2008, 130, 17961.
(c) Wieland, L. C.; Vieira, E. M.; Snapper, M. L.; Hoveyda, A. H. J. Am. Chem. Soc. 2009, 131, 570.
[29] Curti, C.; Battistini, L.; Ranieri, B.; Pelosi, G.; Rassu, G.; Casiraghi, G.; Zanardi, F. J. Org. Chem. 2011, 76, 2248.
[30] Silverio, D. L.; Fu, P.; Carswell, E. L.; Snapper, M. L.; Hoveyda, A. H. Tetrahedron Lett. 2015, 56, 3489.
[31] Rainoldi, G.; Sacchetti, A.; Silvani, A.; Lesma, G. Org. Biomol. Chem. 2016, 14, 7768.
[32] Zhou, L.; Lin, L.; Ji, J.; Xie, M.; Liu, X.; Feng, X. Org. Lett. 2011, 13, 3056.
[33] Guo, Y.-L.; Bai, J.-F.; Peng, L.; Wang, L.-L.; Jia, L.-N.; Luo, X.-Y.; Tian, F.; Xu, X.-Y.; Wang, L.-X. J. Org. Chem. 2012, 77, 8338.
[34] Guo, Y.; Zhang, Y.; Qi, L.; Tian, F.; Wang, L. RSC Adv. 2014, 4, 27286.
[35] Yin, L.; Takada, H.; Kumagai, N.; Shibasaki, M. Angew. Chem., Int. Ed. 2013, 52, 7310.
[36] Nakamura, S.; Yamaji, R.; Hayashi, M. Chem.-Eur. J. 2015, 21, 9615.
[37] Trost, B. M.; Gnanamani, E.; Tracy, J. S.; Kalnmals, C. A. J. Am. Chem. Soc. 2017, 139, 18198.
[38] Wang, Z.-H.; You, Y.; Chen, Y.-Z.; Xu, X.-Y.; Yuan, W.-C. Org. Biomol. Chem. 2018, 16, 1636.
[39] Hitotsuyanagi, Y.; Takeda, E.; Fukaya, H.; Takeya, K. Tet-rahedron Lett. 2008, 49, 7376.
[40] Wang, A.-E.; Huang, P.-Q. Pure Appl. Chem. 2014, 86, 1227.
[41] Tuo, S.-C.; Ye, J.-L.; Wang, A.-E.; Huang, S.-Y.; Huang, P.-Q. Org. Lett. 2011, 13, 5270.
[42] Liu, X.-K.; Ye, J.-L.; Ruan, Y.-P.; Li, Y.-X.; Huang, P.-Q. J. Org. Chem. 2013, 78, 35.
[43] (a) Hanessian, S.; McNaughton-Smith, G. Bioorg. Med. Chem. Lett. 1996, 6, 1567.
(b) Rassu, G.; Carta, P.; Pinna, L.; Battistini, L.; Zanardi, F.; Acquotti, D.; Casiraghi, G. Eur. J. Org. Chem. 1999, 1395.
[44] Miyatake-Ondozabal, H.; Bannwart, L. M.; Gademann, K. Chem. Commun. 2013, 49, 1921.
[45] Wehlauch, R.; Grendelmeier, S. M.; Miyatake-Ondozabal, H.; Sandtorv, A. H.; Scherer, M.; Gademann, K. Org. Lett. 2017, 19, 548.
[46] Ye, J.-L.; Zhang, Y.-F.; Liu, Y.; Zhang, J.-Y.; Ruan, Y.-P.; Huang, P.-Q. Org. Chem. Front. 2015, 2, 697.
[47] Oudeyer, S.; Dudot, B.; Royer, J. Heterocycles 2005, 65, 823.
[48] Ye, J.-L.; Chen, H.; Zhang, Y.-F.; Huang, P.-Q. Org. Chem. Front. 2016, 3, 683.
[49] Ye, J.-L.; Liu, Y.; Yang, Z.-P.; Huang, P.-Q. Chem. Commun. 2016, 52, 561.
[50] Ye, J.-L.; Liu, Y.; Zhang, Y.-F.; Yang, Z.-P.; Huang, P.-Q. Synthesis 2016, 48, 1684.
[51] Yoritate, M.; Takahashi, Y.; Tajima, H.; Ogihara, C.; Yokoyama, T.; Soda, Y.; Oishi, T.; Sato, T.; Chida, N. J. Am. Chem. Soc. 2017, 139, 18386.
[52] Zhou, T.; Gao, J.; Liu, G.; Guan, X.; An, D.; Zhang, S.; Zhang, G. Synlett 2018, 29, 2006.
/
| 〈 |
|
〉 |