

Chinese Journal of Organic Chemistry >
Heterocyclization for the Structures on the Periphery of Py-ropheophorbide and Synthesis of Chlorophyll Derivatives
Received date: 2018-06-30
Revised date: 2018-07-28
Online published: 2018-08-23
Supported by
Project supported by the National Natural Science Foundations of China (No. 21272048) and the University Science and Technology Plan Projects of Shandong Province (No. J15LC51).
In order to expand study and application for the chlorophllous chlorins, pyropheophorbide-a methyl ester was used as a starting material. The chemical modifications and structural transformations along the terminals of N21-N23 axis were carried out to build active electron-accepting functional structures such as aldehyde, α-diketone, enenitrile and ketene moieties. The cyclizations with different electron-sufficient systems were accomplished to synthesize a series of unreported chlorins related to chlorophyll with multiple heterocyclic structures. The chemical structures of new compounds were characterized by elemental analysis, MS, UV-Vis, IR and 1H NMR spectra. Meanwhile the relevant formation process of the heterocyclic ring, the stereochemistry selectivity and the change of the electronic spectrum were discussed.
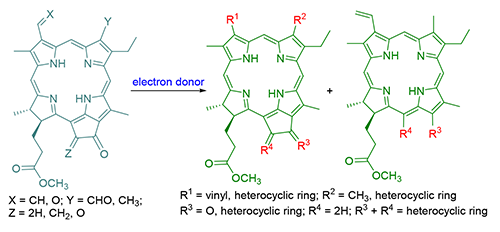
Key words: chlorophyll-a; chlorin; chemical modification; heterocyclization reaction; synthesis
Zhang Zhu , Li Jiazhu , Zhang Shanguo , Wang Zhen , Wang Jinjun . Heterocyclization for the Structures on the Periphery of Py-ropheophorbide and Synthesis of Chlorophyll Derivatives[J]. Chinese Journal of Organic Chemistry, 2018 , 38(12) : 3250 -3259 . DOI: 10.6023/cjoc201806046
[1] (a) Ding, Y.; Zhu, W.-H.; Xie, Y. Chem. Rev. 2017, 117, 2203.
(b) Li, M.; Wei, P.; Ishida, M.; Li, X.; Savage, M.; Guo, R. Angew. Chem., Int. Ed. 2016, 55, 3063.
(c) Machida, S.; Isoda, Y.; Kunieda, M.; Tamiaki, H. Tetrahedron Lett. 2012, 53, 6277.
(d) Li, M.-L.; Peng, X.-J. Acta Chim. Sinica 2016, 74, 959(in Chinese). (李明乐, 彭孝军, 化学学报, 2016, 74, 959.)
[2] (a) Chen, Y. H.; Li, G. L.; Pandey, R. K. Curr. Org. Chem. 2004, 8, 1105.
(b) Wang, J.-J. Chin. J. Org. Chem. 2005, 25, 1353(in Chinese). (王进军, 有机化学, 2005, 25, 1353.)
[3] (a) Zhang, Z.; Jiang, Q.-Y.; Li, J.-Z.; Wang, J.-J. Prog. Chem. 2017, 29, 262(in Chinese). (张珠, 姜齐永, 李家柱, 王进军, 化学进展, 2017, 29, 262.)
(b) Pavlov, V. Y.; Ponomarev, G. V. Chem. Heterocycl. Compd. 2004, 40, 393.
[4] (a) Gao, N.; Wang, Z.; Wu, J.; Liu, C.; Wang, J.-J. Chin. J. Org. Chem. 2016, 36, 580(in Chinese). (高娜, 王振, 武进, 刘超, 王进军, 有机化学, 2016, 36, 580.)
(b) Li, Y.-L.; Li, J.-Z.; Zhang, S.-G.; Wang, J.-J. Chin. J. Org. Chem. 2016, 36, 562(in Chinese). (李彦龙, 李家柱, 张善国, 王进军, 有机化学, 2016, 36, 562.)
[5] (a) Srivatsan, A.; Wang, Y.-F.; Joshi, P.; Sajjad, M.; Chen, Y.-H.; Liu, C.; Thankcppan, K.; Missert, J. R.; Tracy, E.; Morgan, J.; Rigual, N.; Baumann, H.; Pandey, R. K. J. Med. Chem. 2011, 54, 6859.
(b) Wang, J.-J.; Li, J.-Z.; Li, Y.-W.; Jakus, J.; Shim, Y.-K. J. Porphyrins Phthalocyanines 2010, 14, 859.
[6] (a) Li, J.-Z.; Zhang, P.; Yao, N.-N.; Zhao, L.-L.; Wang, J.-J.; Shim, Y.-K. Tetrahedron Lett. 2014, 55, 1086.
(b) Li, J.-Z.; He, N.-L.; Liu, Y.; Zhang, Z.-P.; Zhang, X.; Han, X.-Y.; Gai, Y.-Y.; Liu, Y.-M.; Yin, J.-G.; Wang, J.-J. Dyes Pigm. 2017, 146, 189.
[7] Ji, J.-Y.; Wang, L. M.; Jing, J.-R.; Han, G.-F.; Wang, J. J. Chin. J. Org. Chem. 2007, 27, 493(in Chinese).
[8] (a) Gryshuk, A. L.; Chen, Y.-H.; Potter, W.; Ohulachansky, T.; Oseroff, A.; Pandey, R. K. J. Med. Chem. 2006, 49, 1874.
(b) Liu, Y.-Y.; Wang, L.-M.; Yin, J.-G.; Wu, J.; Liu, C.; Zhang, P.; Wang, J.-J. Chin. J. Org. Chem. 2012, 32, 318(in Chinese). (刘冉冉, 王鲁敏, 殷军港, 武进, 刘超, 张朋, 王进军, 有机化学, 2012, 32, 318.)
(c) Yang, Z.; Wang, Z.; Liu, Y.; Xu, X.-S.; Qi, C.-X.; Wang, J.-J. Chin. J. Org. Chem. 2013, 33, 116(in Chinese). (杨泽, 王振, 刘洋, 徐希森, 祁彩霞, 王进军, 有机化学, 2013, 33, 116.)
[9] (a) Tamaiaki, H.; Monobe, R.; Koizumi, S.; Miyatake, T.; Kinoshita, Y. Tetrahedron:Asymmerty 2013, 24, 966.
(b) Zhang, G.; Potter, W. R.; Camacho, S. H.; Missert, J. R.; Wang, C. S.; Bellnier, D. A.; Henderson, B. W.; Rodgers, M. A. J.; Dougherty, T. J.; Pandey, R. K. J. Med. Chem. 2001, 44, 1540.
[10] (a) Jiang, Q,-Y.; Zhang, Z.; Liu, Y.; Yao, N.-N.; Wang, J.-J. Y. Chin. J. Org. Chem. 2017, 37, 1814(in Chinese). (姜齐永, 张珠, 刘洋, 姚楠楠, 王进军, 有机化学, 2017, 37, 1814.)
(b) Liu, Y.; Wu, H.-Q.; Zhang, X.; Pan, Q.; Wang, X.-M.; Peng, W.; Yin, J.-G.; Li, G.-Z.; Li, J.-Z.; Wang, J.-J. Chem. Pap. 2018, 72, 1089.
[11] Zhan, P.-Y.; Li, D.-F.; Wang, G.-J. Chin. J. Org. Chem. 2008, 28, 2039(in Chinese). (战佩英, 李东风, 王进军, 有机化学, 2008, 28, 2039.)
[12] Luca, L. D.; Giacomelli, G.; Porcheddu, A. J. Org. Chem. 2002, 67, 6272.
[13] (a) Smith, K. M.; Gogg, D. A.; Simpson, D. J. J. Am. Chem. Soc. 1985, 107, 4946.
(b) Liu, Y. M.S. Thesis, Yantai University, Yantai, 2014(in Chinese). (刘洋, 硕士论文, 烟台大学, 烟台, 2014.)
(c) Wu, J.; Yin, J.-G.; Zhang, Q.; Sun, C.-M.; Li, F.-G.; Pei, W.; Wang, J.-J. Chin. J. Org. Chem. 2011, 31, 1653(in Chinese). (武进, 殷军港, 张千, 孙传民, 李付国, 裴文, 王进军, 有机化学, 2011, 31, 1653.)
[14] (a) Wang, J.-J.; Wu, X.-R.; Han, G.-F.; Shim, R.-K. Chin. J. Org. Chem. 2005, 25, 313(in Chinese). (王进军, 邬旭然, 韩光范, 沈荣基, 有机化学, 2005, 25, 313.)
(b) Wu, J.; Yin, J.-G.; Liu, Y.-Y.; Yang, Z.; Wang, Z.; Wang, J.-J. Chin. J. Org. Chem. 2012, 32, 149(in Chinese). (武进, 殷军港, 杨泽, 王振, 王进军, 有机化学, 2012, 32, 149.)
(c) Wang, J.-J.; Ji, J.-Y.; Han, G.-F.; Shim, R.-K.; Mori, A.; Hatsui, T. Chin. J. Org. Chem. 2004, 24, 53(in Chinese). (王进军, 纪建业, 韩光范, 沈荣基, Mori, A.; Hatsui, T., 有机化学, 2004, 24, 53.)
/
| 〈 |
|
〉 |